当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Bone Marrow Myeloid Cells Regulate Myeloid-Biased Hematopoietic Stem Cells via a Histamine-Dependent Feedback Loop.
Cell Stem Cell ( IF 23.9 ) Pub Date : 2017-Dec-07 , DOI: 10.1016/j.stem.2017.11.003 Xiaowei Chen 1 , Huan Deng 2 , Michael J Churchill 3 , Larry L Luchsinger 4 , Xing Du 3 , Timothy H Chu 5 , Richard A Friedman 6 , Moritz Middelhoff 5 , Hongxu Ding 7 , Yagnesh H Tailor 5 , Alexander L E Wang 5 , Haibo Liu 5 , Zhengchuan Niu 8 , Hongshan Wang 8 , Zhengyu Jiang 5 , Simon Renders 9 , Siu-Hong Ho 10 , Spandan V Shah 10 , Pavel Tishchenko 10 , Wenju Chang 8 , Theresa C Swayne 11 , Laura Munteanu 11 , Andrea Califano 7 , Ryota Takahashi 5 , Karan K Nagar 5 , Bernhard W Renz 12 , Daniel L Worthley 13 , C Benedikt Westphalen 14 , Yoku Hayakawa 15 , Samuel Asfaha 16 , Florence Borot 3 , Chyuan-Sheng Lin 17 , Hans-Willem Snoeck 18 , Siddhartha Mukherjee 3 , Timothy C Wang 5
Cell Stem Cell ( IF 23.9 ) Pub Date : 2017-Dec-07 , DOI: 10.1016/j.stem.2017.11.003 Xiaowei Chen 1 , Huan Deng 2 , Michael J Churchill 3 , Larry L Luchsinger 4 , Xing Du 3 , Timothy H Chu 5 , Richard A Friedman 6 , Moritz Middelhoff 5 , Hongxu Ding 7 , Yagnesh H Tailor 5 , Alexander L E Wang 5 , Haibo Liu 5 , Zhengchuan Niu 8 , Hongshan Wang 8 , Zhengyu Jiang 5 , Simon Renders 9 , Siu-Hong Ho 10 , Spandan V Shah 10 , Pavel Tishchenko 10 , Wenju Chang 8 , Theresa C Swayne 11 , Laura Munteanu 11 , Andrea Califano 7 , Ryota Takahashi 5 , Karan K Nagar 5 , Bernhard W Renz 12 , Daniel L Worthley 13 , C Benedikt Westphalen 14 , Yoku Hayakawa 15 , Samuel Asfaha 16 , Florence Borot 3 , Chyuan-Sheng Lin 17 , Hans-Willem Snoeck 18 , Siddhartha Mukherjee 3 , Timothy C Wang 5
Affiliation
|
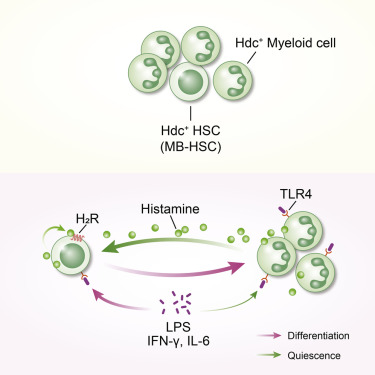
Myeloid-biased hematopoietic stem cells (MB-HSCs) play critical roles in recovery from injury, but little is known about how they are regulated within the bone marrow niche. Here we describe an auto-/paracrine physiologic circuit that controls quiescence of MB-HSCs and hematopoietic progenitors marked by histidine decarboxylase (Hdc). Committed Hdc+ myeloid cells lie in close anatomical proximity to MB-HSCs and produce histamine, which activates the H2 receptor on MB-HSCs to promote their quiescence and self-renewal. Depleting histamine-producing cells enforces cell cycle entry, induces loss of serial transplant capacity, and sensitizes animals to chemotherapeutic injury. Increasing demand for myeloid cells via lipopolysaccharide (LPS) treatment specifically recruits MB-HSCs and progenitors into the cell cycle; cycling MB-HSCs fail to revert into quiescence in the absence of histamine feedback, leading to their depletion, while an H2 agonist protects MB-HSCs from depletion after sepsis. Thus, histamine couples lineage-specific physiological demands to intrinsically primed MB-HSCs to enforce homeostasis.
中文翻译:

骨髓髓系细胞通过组胺依赖性反馈回路调节偏向髓系的造血干细胞。
偏向骨髓的造血干细胞 (MB-HSC) 在损伤恢复中起着关键作用,但人们对它们在骨髓生态位中的调节方式知之甚少。在这里,我们描述了一种自体/旁分泌生理回路,它控制 MB-HSC 和以组氨酸脱羧酶 (Hdc) 为标记的造血祖细胞的静止。定型 Hdc +髓样细胞在解剖学上靠近 MB-HSC,并产生组胺,激活 H 2MB-HSCs 上的受体以促进它们的静止和自我更新。耗尽产生组胺的细胞会强制进入细胞周期,导致连续移植能力丧失,并使动物对化疗损伤敏感。通过脂多糖 (LPS) 处理增加对骨髓细胞的需求,特异性地将 MB-HSC 和祖细胞募集到细胞周期中;在没有组胺反馈的情况下,循环的 MB-HSC 无法恢复静止状态,导致它们耗尽,而 H 2激动剂可保护 MB-HSC 在败血症后免于耗尽。因此,组胺将谱系特异性生理需求与内在启动的 MB-HSC 相结合,以加强体内平衡。
更新日期:2017-12-01
中文翻译:

骨髓髓系细胞通过组胺依赖性反馈回路调节偏向髓系的造血干细胞。
偏向骨髓的造血干细胞 (MB-HSC) 在损伤恢复中起着关键作用,但人们对它们在骨髓生态位中的调节方式知之甚少。在这里,我们描述了一种自体/旁分泌生理回路,它控制 MB-HSC 和以组氨酸脱羧酶 (Hdc) 为标记的造血祖细胞的静止。定型 Hdc +髓样细胞在解剖学上靠近 MB-HSC,并产生组胺,激活 H 2MB-HSCs 上的受体以促进它们的静止和自我更新。耗尽产生组胺的细胞会强制进入细胞周期,导致连续移植能力丧失,并使动物对化疗损伤敏感。通过脂多糖 (LPS) 处理增加对骨髓细胞的需求,特异性地将 MB-HSC 和祖细胞募集到细胞周期中;在没有组胺反馈的情况下,循环的 MB-HSC 无法恢复静止状态,导致它们耗尽,而 H 2激动剂可保护 MB-HSC 在败血症后免于耗尽。因此,组胺将谱系特异性生理需求与内在启动的 MB-HSC 相结合,以加强体内平衡。


























 京公网安备 11010802027423号
京公网安备 11010802027423号