当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rational design of asymmetric benzodithiophene based photovoltaic polymers for efficient solar cells†
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-11-30 00:00:00 , DOI: 10.1039/c7ta09736k Tingting Zhu 1, 2, 3, 4 , Deyu Liu 4, 5, 6, 7, 8 , Kaili Zhang 1, 2, 3, 4 , Yonghai Li 4, 5, 6, 7, 8 , Zhe Liu 1, 2, 3, 4 , Xudong Gao 1, 2, 3, 4 , Xichang Bao 4, 5, 6, 7, 8 , Mingliang Sun 1, 2, 3, 4 , Renqiang Yang 4, 5, 6, 7, 8
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2017-11-30 00:00:00 , DOI: 10.1039/c7ta09736k Tingting Zhu 1, 2, 3, 4 , Deyu Liu 4, 5, 6, 7, 8 , Kaili Zhang 1, 2, 3, 4 , Yonghai Li 4, 5, 6, 7, 8 , Zhe Liu 1, 2, 3, 4 , Xudong Gao 1, 2, 3, 4 , Xichang Bao 4, 5, 6, 7, 8 , Mingliang Sun 1, 2, 3, 4 , Renqiang Yang 4, 5, 6, 7, 8
Affiliation
|
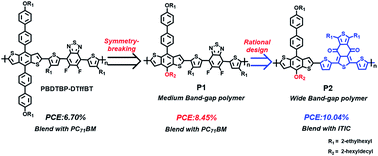
Extending π-conjugation in the benzodithiophene (BDT) side chains has been proven useful to improve the efficiencies of the BDT-based polymer solar cells (PSCs). Herein, combined with a symmetry-breaking strategy of a BDT unit, we further designed a new asymmetric 1D–2D (one dimensional–two dimensional) monomer asy-BDTBP with an alkoxyl group as the 1D part and a π-extending alkoxybiphenyl as the 2D substituted group. Medium band-gap donor–acceptor (D–A) conjugated polymer P1 was synthesized with asy-BDTBP and 4,7-di(4-(2-ethylhexyl)-2-thienyl)-5,6-difluoro-2,1,3-benzothiadiazole (DTffBT) as the donor and acceptor unit, respectively. Encouragingly, P1 blended with PC71BM exhibited an obviously enhanced power conversion efficiency (PCE) compared to the reported symmetric analogue PBDTBP–DTffBT (6.70%). The PCE increased to 8.45% with an open-circuit voltage (VOC) of 0.838 V, a short-circuit current density (JSC) of 14.35 mA cm−2 and a fill factor (FF) of 70.27%. However, P1 coupled with a classical non-fullerene acceptor ITIC revealed a relatively poor efficiency of 6.35% due to the bad complementarity of absorption spectra. To match the absorption of ITIC, a wide band-gap D–A polymer P2 was further designed with a weak electron-withdrawing group benzo[1,2-c:4,5-c′]dithiophene-4,8-dione (BDD) instead of DTffBT as the acceptor unit. As a result, P2 possessed a complementary absorption spectrum with ITIC, and the resulting devices presented an excellent photovoltaic performance. The optimal efficiency boosted to 10.04% with VOC of 0.873 V, JSC of 17.60 mA cm−2 and FF of 65.37%. This work demonstrates the great potential of asymmetric BDTs for high efficient PSCs and the importance of the rational design of polymers for different types of PSCs.
中文翻译:

用于高效太阳能电池的不对称基于苯并二噻吩的光伏聚合物的合理设计†
已证明在苯并二噻吩(BDT)侧链中扩展π共轭可提高基于BDT的聚合物太阳能电池(PSC)的效率。在此,结合BDT单元的对称性破缺策略,我们进一步设计了一种新的不对称1D–2D(一维–二维)单体asy-BDTBP,其中烷氧基为1D部分,而π扩展的烷氧基联苯为2D取代基。中等带隙的供体-受体(DA)共轭聚合物P1与asy-BDTBP和4,7-二(4-(2-乙基己基)-2-噻吩基)-5,6-二氟-2,1合成,3-苯并噻二唑(DTffBT)分别作为供体和受体单元。令人鼓舞的是,P1与PC 71融合在一起与报道的对称模拟PBDTBP–DTffBT(6.70%)相比,BM具有明显增强的功率转换效率(PCE)。当开路电压(V OC)为0.838 V,短路电流密度(J SC)为14.35 mA cm -2且填充系数(FF)为70.27%时,PCE增至8.45 %。然而,由于吸收光谱的互补性差,P1与经典的非富勒烯受体ITIC结合显示出6.35%的相对较差的效率。为了与ITIC的吸收相匹配,还设计了具有较弱吸电子基团苯并[1,2- c:4,5- c]的宽带隙D–A聚合物P2。']二噻吩-4,8-二酮(BDD)代替DTffBT作为受体单元。结果,P2具有与ITIC互补的吸收光谱,并且所得的器件表现出优异的光伏性能。当V OC为0.873 V,J SC为17.60 mA cm -2且FF为65.37%时,最佳效率提高到10.04 %。这项工作证明了不对称BDT对于高效PSC的巨大潜力,以及为不同类型的PSC合理设计聚合物的重要性。
更新日期:2017-11-30
中文翻译:

用于高效太阳能电池的不对称基于苯并二噻吩的光伏聚合物的合理设计†
已证明在苯并二噻吩(BDT)侧链中扩展π共轭可提高基于BDT的聚合物太阳能电池(PSC)的效率。在此,结合BDT单元的对称性破缺策略,我们进一步设计了一种新的不对称1D–2D(一维–二维)单体asy-BDTBP,其中烷氧基为1D部分,而π扩展的烷氧基联苯为2D取代基。中等带隙的供体-受体(DA)共轭聚合物P1与asy-BDTBP和4,7-二(4-(2-乙基己基)-2-噻吩基)-5,6-二氟-2,1合成,3-苯并噻二唑(DTffBT)分别作为供体和受体单元。令人鼓舞的是,P1与PC 71融合在一起与报道的对称模拟PBDTBP–DTffBT(6.70%)相比,BM具有明显增强的功率转换效率(PCE)。当开路电压(V OC)为0.838 V,短路电流密度(J SC)为14.35 mA cm -2且填充系数(FF)为70.27%时,PCE增至8.45 %。然而,由于吸收光谱的互补性差,P1与经典的非富勒烯受体ITIC结合显示出6.35%的相对较差的效率。为了与ITIC的吸收相匹配,还设计了具有较弱吸电子基团苯并[1,2- c:4,5- c]的宽带隙D–A聚合物P2。']二噻吩-4,8-二酮(BDD)代替DTffBT作为受体单元。结果,P2具有与ITIC互补的吸收光谱,并且所得的器件表现出优异的光伏性能。当V OC为0.873 V,J SC为17.60 mA cm -2且FF为65.37%时,最佳效率提高到10.04 %。这项工作证明了不对称BDT对于高效PSC的巨大潜力,以及为不同类型的PSC合理设计聚合物的重要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号