Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2017-11-29 , DOI: 10.1016/j.jfluchem.2017.11.012 Wei Li , Henri Groult , Olaf J. Borkiewicz , Damien Dambournet
|
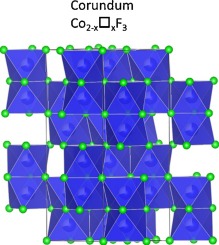
Metal fluorides are potential candidates as electrode materials for rechargeable batteries. During the electrode fabrication, however, thermal treatments can cause decomposition of thermally instable compounds. Here, we showed that during the electrode processing of CoF3, the carbon and PVDF additives act as a protective layer that prevents CoF3 to readily decompose into CoF2 (rutile). We found that instead, it decomposes into an intermediate phase with a corundum like structure featuring Co vacancies, i.e., Co1.26IICo0.16III□0.58F3 where □ represents the vacancies. The structural analysis was possible owing to the use of the pair distribution function.
PACS(optional, as per journal): 75.40.-s; 71.20.LP
中文翻译:

电池电极加工过程中CoF 3的分解
金属氟化物可能是可再充电电池的电极材料。然而,在电极制造期间,热处理会引起热不稳定化合物的分解。在此,我们表明,在CoF 3的电极加工过程中,碳和PVDF添加剂起着保护层的作用,可防止CoF 3容易分解成CoF 2(金红石)。我们发现,它分解成具有刚玉状结构的中间相,其结构具有Co空位,即Co 1.26 II Co 0.16 III □ 0.58 F 3其中□表示空缺。由于使用了配对分布函数,因此可以进行结构分析。
PACS(可选,根据日志): 75.40.-s;71.20.LP



























 京公网安备 11010802027423号
京公网安备 11010802027423号