当前位置:
X-MOL 学术
›
Macromol. Rapid Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Kinetics of Thermal Polymerization Can Be Studied during Continuous Cooling
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2017-11-29 , DOI: 10.1002/marc.201700624 Tatsiana Liavitskaya 1 , Sergey Vyazovkin 1
Macromolecular Rapid Communications ( IF 4.6 ) Pub Date : 2017-11-29 , DOI: 10.1002/marc.201700624 Tatsiana Liavitskaya 1 , Sergey Vyazovkin 1
Affiliation
|
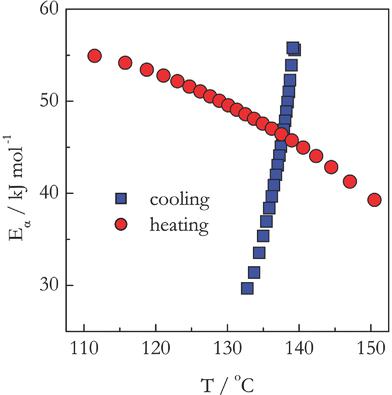
It is demonstrated that differential scanning calorimetry can measure the kinetics of the thermally initiated polymerization during continuous cooling. The measurements are accomplished by switching from fast heating to much slower cooling. The study is exemplified by crosslinking polymerization (curing) of diglycidyl ether of bisphenol A epoxy and m‐phenylenediamine taken in stoichiometric and nonstoichiometric ratios and measured under heating and cooling conditions. An advanced isoconversional method reveals that the reaction in the nonstoichiometric system follows the kinetics of the single‐step type. Its activation energy is constant and the same for heating and cooling conditions. The stoichiometric system exhibits the multistep kinetics characterized by the dependencies of the activation energy on temperature that differ qualitatively for cooling and heating runs. The discovered differences emphasize the need for further systematic studies of the thermal polymerization during continuous cooling.
中文翻译:

连续冷却过程中热聚合动力学的研究
结果表明,差示扫描量热法可以测量连续冷却过程中热引发聚合反应的动力学。通过从快速加热切换到慢得多的冷却来完成测量。该研究是通过交联的双酚A环氧树脂和二缩水甘油醚的聚合(固化)例举米苯二胺以化学计量比和非化学计量比计,并在加热和冷却条件下测量。一种先进的等转化方法表明,非化学计量系统中的反应遵循一步法的动力学。它的活化能是恒定的,并且在加热和冷却条件下都相同。化学计量系统表现出多步动力学,其特征在于活化能对温度的依赖性在冷却和加热过程中在质量上有所不同。发现的差异强调了在连续冷却过程中需要对热聚合进行进一步系统研究的必要性。
更新日期:2017-11-29
中文翻译:

连续冷却过程中热聚合动力学的研究
结果表明,差示扫描量热法可以测量连续冷却过程中热引发聚合反应的动力学。通过从快速加热切换到慢得多的冷却来完成测量。该研究是通过交联的双酚A环氧树脂和二缩水甘油醚的聚合(固化)例举米苯二胺以化学计量比和非化学计量比计,并在加热和冷却条件下测量。一种先进的等转化方法表明,非化学计量系统中的反应遵循一步法的动力学。它的活化能是恒定的,并且在加热和冷却条件下都相同。化学计量系统表现出多步动力学,其特征在于活化能对温度的依赖性在冷却和加热过程中在质量上有所不同。发现的差异强调了在连续冷却过程中需要对热聚合进行进一步系统研究的必要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号