当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Allosteric Effects in Ethylene Polymerization Catalysis. Enhancement of Performance of Phosphine-Phosphinate and Phosphine-Phosphonate Palladium Alkyl Catalysts by Remote Binding of B(C6F5)3
Organometallics ( IF 2.8 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00815 Alison M. Johnson 1 , Nathan D. Contrella 1 , Jessica R. Sampson 1 , Mingfang Zheng 1 , Richard F. Jordan 1
Organometallics ( IF 2.8 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.organomet.7b00815 Alison M. Johnson 1 , Nathan D. Contrella 1 , Jessica R. Sampson 1 , Mingfang Zheng 1 , Richard F. Jordan 1
Affiliation
|
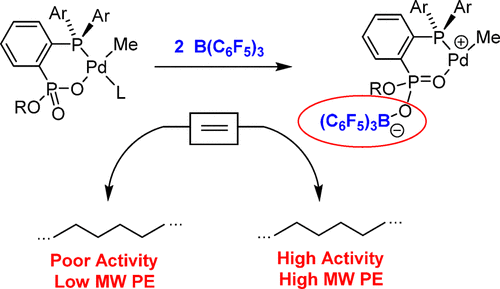
Remote binding of B(C6F5)3 to (PPO)PdMeL (L = pyridine or lutidine) or {(PPO)PdMe}2 ethylene polymerization catalysts that contain phosphine-arenephosphinate or phosphine-arenephosphonate ligands (PPO– = [1-PAr2-2-PR′O2-Ph]−: Ar = R′ = Ph (1a); Ar = Ph, R′ = OEt (1b); Ar = Ph, R′ = OiPr (1c); Ar = 2-OMe-Ph, R′ = OiPr (1d)) significantly increases the catalyst activity and the molecular weight of the polyethylene (PE) product. In the most favorable case, in situ conversion of (1d)PdMe(py) to the base-free adduct {1d·B(C6F5)3}PdMe increases the ethylene polymerization activity from 9.8 to 5700 kg mol–1 h–1 and the Mn of the PE product from 9030 to 99 200 Da (80 °C, 410 psi). X-ray structural data, trends in ligand lability, and comparative studies of BF3 activation suggest that these allosteric effects are primarily electronic in origin. The B(C6F5)3 binding enhances the chain growth rate (Rgrowth) by increasing the degree of positive charge on the Pd center. This effect does not result in the large increase in the chain transfer rate (Rtransfer) and concomitant reduction in PE molecular weight seen in previous studies of analogous (PO)PdRL catalysts that contain phosphine-arenesulfonate ligands, because of the operation of a dissociative chain transfer process, which is inhibited by the increased charge at Pd.
中文翻译:

乙烯聚合催化中的变构作用。通过远程键合B(C 6 F 5)3增强膦膦酸酯和膦膦酸酯钯烷基催化剂的性能
B(C 6 F 5)3与(PPO)PdMeL(L =吡啶或二甲基吡啶)或{(PPO)PdMe} 2的乙烯聚合催化剂的远程结合,这些催化剂包含膦-芳烃次膦酸酯或膦-芳烃膦酸酯配体(PPO – = [1 -PAr 2 -2-PR'O 2 -Ph] -:Ar = R'= Ph(1a); Ar = Ph,R'= OEt(1b); Ar = Ph,R'= O i Pr(1c) ; Ar = 2-OMe-Ph,R'= O i Pr(1d))显着提高了催化剂活性和聚乙烯(PE)产品的分子量。在最有利的情况下,原位(转化1D)PDME(PY),以游离碱加合物{ 1D ·B(C 6 ˚F 5)3 } PDME增加了从9.8乙烯聚合活性来5700千克摩尔-1 ħ -1和中号Ñ的从9030到99200 Da(80°C,410 psi)的PE产品。X射线结构数据,配体不稳定性趋势以及BF 3活化的比较研究表明,这些变构作用主要是电子起源的。B(C 6 F 5)3结合提高链增长速率(R增长),以增加Pd中心的正电荷程度。这种作用不会导致链转移速率(R transfer)大幅增加,也不会导致PE分子量的降低,这是因为以前的研究中,由于膦基芳烃磺酸盐配位基的存在,类似的(PO)PdRL催化剂含有膦-芳烃磺酸盐配体。离解链转移过程,其受Pd电荷增加的抑制。
更新日期:2017-11-29
中文翻译:

乙烯聚合催化中的变构作用。通过远程键合B(C 6 F 5)3增强膦膦酸酯和膦膦酸酯钯烷基催化剂的性能
B(C 6 F 5)3与(PPO)PdMeL(L =吡啶或二甲基吡啶)或{(PPO)PdMe} 2的乙烯聚合催化剂的远程结合,这些催化剂包含膦-芳烃次膦酸酯或膦-芳烃膦酸酯配体(PPO – = [1 -PAr 2 -2-PR'O 2 -Ph] -:Ar = R'= Ph(1a); Ar = Ph,R'= OEt(1b); Ar = Ph,R'= O i Pr(1c) ; Ar = 2-OMe-Ph,R'= O i Pr(1d))显着提高了催化剂活性和聚乙烯(PE)产品的分子量。在最有利的情况下,原位(转化1D)PDME(PY),以游离碱加合物{ 1D ·B(C 6 ˚F 5)3 } PDME增加了从9.8乙烯聚合活性来5700千克摩尔-1 ħ -1和中号Ñ的从9030到99200 Da(80°C,410 psi)的PE产品。X射线结构数据,配体不稳定性趋势以及BF 3活化的比较研究表明,这些变构作用主要是电子起源的。B(C 6 F 5)3结合提高链增长速率(R增长),以增加Pd中心的正电荷程度。这种作用不会导致链转移速率(R transfer)大幅增加,也不会导致PE分子量的降低,这是因为以前的研究中,由于膦基芳烃磺酸盐配位基的存在,类似的(PO)PdRL催化剂含有膦-芳烃磺酸盐配体。离解链转移过程,其受Pd电荷增加的抑制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号