当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigating Pharmacological Targeting of G-Quadruplexes in the Human Malaria Parasite
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00964 Mohammad Anas 1 , Richa Sharma 1 , V. Dhamodharan 2 , P. I. Pradeepkumar 2 , Ashan Manhas 1 , Kumkum Srivastava 1, 3 , Shakil Ahmed 3, 4 , Niti Kumar 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2017-12-14 00:00:00 , DOI: 10.1021/acs.biochem.7b00964 Mohammad Anas 1 , Richa Sharma 1 , V. Dhamodharan 2 , P. I. Pradeepkumar 2 , Ashan Manhas 1 , Kumkum Srivastava 1, 3 , Shakil Ahmed 3, 4 , Niti Kumar 1, 3
Affiliation
|
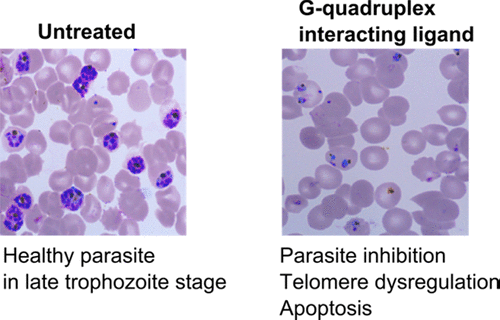
The unique occurrence of G-quadruplexes in the AT-rich genome of human malaria parasite Plasmodium falciparum provides hints about their critical roles in parasite survival, pathogenesis, and host immune evasion. An intriguing question is whether these noncanonical structures can serve as molecular targets for small molecule-based interventions against malaria. In this study, we have investigated the pharmacological targeting of G-quadruplexes for parasite inhibition. We observed that bisquinolinium derivatives of 1,8-naphthyridine and pyridine affected the stability and molecular recognition properties of G-quadruplexes in telomeric and subtelomeric regions in P. falciparum. Parasite inhibition and cytotoxicity assays revealed that these ligands effectively inhibit parasite growth with minimal toxic effects in human cells. G-quadruplex interacting ligands caused degeneration and shortening of parasite telomeres. Ligand-induced perturbations in telomere homeostasis also affected transcriptional state of the subtelomeric region harboring antigenic variation genes. Taken together, our results suggest that quadruplex–ligand interaction disturbs telomeric/subtelomeric chromatin organization and induces DNA damage that consequently leads to parasite death. Our findings also draw attention to the striking differences in telomere dynamics in the protozoan parasite and human host that can be exploited for selective targeting of the telomeric quadruplex of the parasite as a potential antimalarial strategy.
中文翻译:

调查人类疟疾寄生虫中G四联体的药理靶向。
G-四链体在人类疟疾寄生虫恶性疟原虫的富含AT的基因组中的独特出现提供了有关它们在寄生虫存活,发病机理和宿主免疫逃避中的关键作用的暗示。一个有趣的问题是这些非规范结构是否可以作为基于小分子的疟疾干预措施的分子靶标。在这项研究中,我们研究了G-四链体对寄生虫抑制的药理作用。我们观察到1,8-萘啶和吡啶的双喹啉鎓衍生物影响恶性疟原虫端粒和亚端粒区域G-四链体的稳定性和分子识别特性。。寄生虫抑制和细胞毒性试验表明,这些配体有效抑制了寄生虫的生长,对人体细胞的毒性作用极小。G四联体相互作用的配体引起寄生虫端粒的变性和缩短。端粒稳态中的配体诱导的扰动也影响具有抗原变异基因的亚端粒区域的转录状态。两者合计,我们的结果表明,四链体-配体相互作用会干扰端粒/亚端粒染色质的组织,并诱导DNA损伤,从而导致寄生虫死亡。我们的发现还引起人们对原生动物寄生虫和人类宿主端粒动力学的显着差异的关注,这些差异可被用于选择性靶向该寄生虫的端粒四聚体,作为潜在的抗疟策略。
更新日期:2017-12-14
中文翻译:

调查人类疟疾寄生虫中G四联体的药理靶向。
G-四链体在人类疟疾寄生虫恶性疟原虫的富含AT的基因组中的独特出现提供了有关它们在寄生虫存活,发病机理和宿主免疫逃避中的关键作用的暗示。一个有趣的问题是这些非规范结构是否可以作为基于小分子的疟疾干预措施的分子靶标。在这项研究中,我们研究了G-四链体对寄生虫抑制的药理作用。我们观察到1,8-萘啶和吡啶的双喹啉鎓衍生物影响恶性疟原虫端粒和亚端粒区域G-四链体的稳定性和分子识别特性。。寄生虫抑制和细胞毒性试验表明,这些配体有效抑制了寄生虫的生长,对人体细胞的毒性作用极小。G四联体相互作用的配体引起寄生虫端粒的变性和缩短。端粒稳态中的配体诱导的扰动也影响具有抗原变异基因的亚端粒区域的转录状态。两者合计,我们的结果表明,四链体-配体相互作用会干扰端粒/亚端粒染色质的组织,并诱导DNA损伤,从而导致寄生虫死亡。我们的发现还引起人们对原生动物寄生虫和人类宿主端粒动力学的显着差异的关注,这些差异可被用于选择性靶向该寄生虫的端粒四聚体,作为潜在的抗疟策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号