当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alteration in MicroRNA Expression Governs the Nature and Timing of Cellular Fate Commitment
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acschemneuro.7b00423 Dola Sengupta 1 , Sandip Kar 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acschemneuro.7b00423 Dola Sengupta 1 , Sandip Kar 1
Affiliation
|
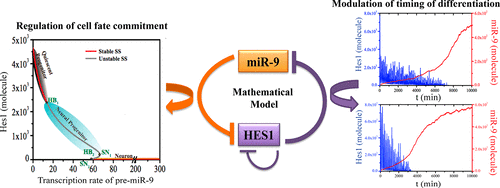
In the central nervous system, the expression level of transcriptional repressor Hes1 (hairy and enhancer of split-1) tightly controls the alternative cell fate commitment during differentiation as well as the time required for such cellular transitions. A microRNA, miR-9, that interacts with Hes1 in a mutually antagonistic manner, influences both the process of lineage specification and timing of differentiation significantly, but the impact of the miR-9 in guiding these events still remains poorly understood. Here, we proposed a stochastic mathematical model of the miR-9/Hes1 double-negative feedback interaction network that at the outset shows how alternative cell fate such as quiescence, progenitor, and neuronal states can be accomplished through fine-tuning the Hes1 dynamics by altering the expression level of miR-9. The model simulations further foretell a correlated variation of the period of oscillation of Hes1, and the time delay observed between Hes1 mRNA and protein as the transcription rate of miR-9 increases during the neural progenitor state attainment. Importantly, the model simulations aided by the systematic sensitivity analysis predict that the timing of differentiation to the neuronal state crucially depends on the negative regulators (miR-9 and Hes6) of the Hes1. Our results indicate that miR-9/Hes1 interaction network can be effectively exploited for an efficient and well-timed neuronal transformation.
中文翻译:

MicroRNA表达的改变控制着细胞命运承诺的性质和时机
在中枢神经系统中,转录阻遏物Hes1(有毛的和split-1的增强子)的表达水平紧密控制着分化过程中替代性的细胞命运,以及这种细胞过渡所需的时间。与hes1相互作用的microRNA miR-9以相互拮抗的方式显着影响谱系指定过程和分化时间,但对miR-9指导这些事件的影响仍然知之甚少。在这里,我们提出了miR-9 / Hes1双负反馈相互作用网络的随机数学模型,该模型首先显示了如何通过对Hes1动力学进行微调来实现替代细胞命运,例如静止,祖细胞和神经元状态。改变miR-9的表达水平。在神经祖细胞状态获得过程中,随着miR-9转录速率的增加,Hes1 mRNA和蛋白质含量增加。重要的是,在系统敏感性分析的帮助下,模型仿真预测,分化为神经元状态的时机关键取决于Hes1的负调节因子(miR-9和Hes6)。我们的结果表明,可以有效利用miR-9 / Hes1相互作用网络进行高效且及时的神经元转化。
更新日期:2017-11-28
中文翻译:

MicroRNA表达的改变控制着细胞命运承诺的性质和时机
在中枢神经系统中,转录阻遏物Hes1(有毛的和split-1的增强子)的表达水平紧密控制着分化过程中替代性的细胞命运,以及这种细胞过渡所需的时间。与hes1相互作用的microRNA miR-9以相互拮抗的方式显着影响谱系指定过程和分化时间,但对miR-9指导这些事件的影响仍然知之甚少。在这里,我们提出了miR-9 / Hes1双负反馈相互作用网络的随机数学模型,该模型首先显示了如何通过对Hes1动力学进行微调来实现替代细胞命运,例如静止,祖细胞和神经元状态。改变miR-9的表达水平。在神经祖细胞状态获得过程中,随着miR-9转录速率的增加,Hes1 mRNA和蛋白质含量增加。重要的是,在系统敏感性分析的帮助下,模型仿真预测,分化为神经元状态的时机关键取决于Hes1的负调节因子(miR-9和Hes6)。我们的结果表明,可以有效利用miR-9 / Hes1相互作用网络进行高效且及时的神经元转化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号