Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2017-05-31 , DOI: 10.1016/j.jfluchem.2017.05.012 Matthew C. Leclerc , Jason G. Da Gama , Bulat M. Gabidullin , R. Tom Baker
|
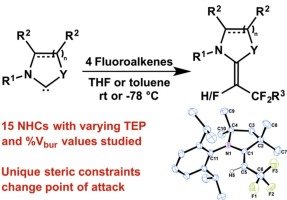
The fundamental reactivity leading to N-heterocyclic fluoroalkene adducts is explored in detail, featuring a total of 15 N-heterocyclic carbenes (NHCs) with various electronic and steric environments. The activity of these carbenes towards tetrafluoroethylene (TFE), hexafluoropropene (HFP), trifluoroethylene (HTFE) and vinylidene fluoride (VDF) is assessed in THF and toluene. Attempts were made to correlate the observed reactivity with electronic (Tolman Electronic Parameters) and steric (% buried volume) parameters unique to each NHC, but a trend has yet to be fully determined. However, the unique steric constraints of a cyclic (alkyl)(amino)carbene (CAAC) were shown to modify the initial point of nucleophilic attack on HTFE, providing selective transformation to a different adduct than has been observed to date with all reactions involving this fluoroalkene.
中文翻译:

仔细研究N杂环卡宾与氟代烯烃之间的反应性
详细探讨了导致N-杂环氟烯烃加合物的基本反应性,共15 N-具有各种电子和空间环境的杂环卡宾(NHC)。在THF和甲苯中评估了这些羧苯对四氟乙烯(TFE),六氟丙烯(HFP),三氟乙烯(HTFE)和偏二氟乙烯(VDF)的活性。尝试将观察到的反应性与每个NHC特有的电子(托曼电子参数)和空间(掩埋体积百分比)参数相关联,但是趋势尚未完全确定。但是,环状(烷基)(氨基)碳烯(CAAC)的独特空间约束条件显示其可改变对HTFE的亲核进攻的起始点,从而提供了选择性转化为不同于迄今为止涉及该反应的所有加合物的选择性加合物。氟烯烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号