Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2017-07-23 , DOI: 10.1016/j.jfluchem.2017.07.006 Nadia O. Ilchenko , Kálmán J. Szabó
|
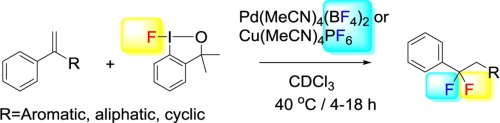
α,α’-Disubstituted styrenes undergo a difluorination-rearrangement reaction with fluoro-benzoiodoxole reagent 1. The reaction is catalyzed by Pd(MeCN)4(BF4)2 and Cu(MeCN)4PF6. We have studied the rearrangement of α,α’-diaryl substituted styrenes, in which the aryl groups have different electronic character. In the case of α-aryl, α’-alkyl substituted styrenes, the aryl substituent has a higher migratory aptitude than the alkyl group. We have also extended the reactions to cycloalkyl styrenes, which underwent interesting ring contraction/expansion reactions. The regioselectivity of the migration can be explained on the basis of the formation of a phenonium intermediate.
中文翻译:

使用氟代苯并恶唑试剂对α,α'-二取代苯乙烯进行双季胺氟化。α-取代基的迁移能力
α,α′-二取代的苯乙烯与氟-苯并碘代恶唑试剂1进行二氟化重排反应。该反应由Pd(MeCN)4(BF 4)2和Cu(MeCN)4 PF 6催化。我们已经研究了α,α'-二芳基取代的苯乙烯的重排,其中芳基具有不同的电子特性。在α-芳基,α'-烷基取代的苯乙烯的情况下,芳基取代基具有比烷基更高的迁移能力。我们还将反应扩展到环烷基苯乙烯,该环烷基苯乙烯经历了有趣的环收缩/扩环反应。迁移的区域选择性可以基于a中间体的形成来解释。


























 京公网安备 11010802027423号
京公网安备 11010802027423号