当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic studies of base-catalysed lignin depolymerisation in dimethyl carbonate
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-24 00:00:00 , DOI: 10.1039/c7gc03110f Saumya Dabral 1, 2, 3, 4 , Julien Engel 1, 2, 3, 4 , Jakob Mottweiler 1, 2, 3, 4 , Stephanie S. M. Spoehrle 1, 2, 3, 4, 5 , Ciaran W. Lahive 5, 6, 7, 8 , Carsten Bolm 1, 2, 3, 4
Green Chemistry ( IF 9.8 ) Pub Date : 2017-11-24 00:00:00 , DOI: 10.1039/c7gc03110f Saumya Dabral 1, 2, 3, 4 , Julien Engel 1, 2, 3, 4 , Jakob Mottweiler 1, 2, 3, 4 , Stephanie S. M. Spoehrle 1, 2, 3, 4, 5 , Ciaran W. Lahive 5, 6, 7, 8 , Carsten Bolm 1, 2, 3, 4
Affiliation
|
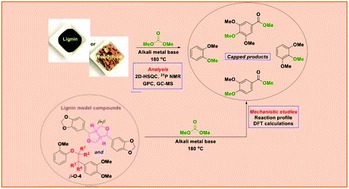
The depleting fossil reservoirs have stimulated global research initiatives on renewable lignin feedstocks as sustainable alternatives to petroleum-derived aromatics. Base-catalysed depolymerisation (BCD) is regarded as an economical and efficient approach for the valorisation of technical lignins. The major limiting factor encountered during this process is the re-condensation of the formed phenolic products, which results in lower monomer yields. To diminish these side reactions, we selected alkali earth metal catalysts in dimethyl carbonate (DMC) to produce methylated phenol derivatives as the final products. Herein, we demonstrate for the first time a base-promoted depolymerisation process affording low-molecular weight oils in high yields (52–67 wt%) wherein the employed bases are used in truly catalytic quantities (with catalyst loadings of around 5 mol%). The general applicability of this methodology was proved on four different lignin samples (1 Kraft, 3 organosolv) using caesium carbonate and lithium tert-butoxide as catalysts. The 2D NMR studies on the post-reaction lignin samples showed a similar degradation of the major lignin linkages for both bases. A difference in the reduction of phenolic moieties was revealed by quantitative 31P NMR analysis. Furthermore, GPC analysis demonstrated a significant shift towards lower mass fragments for the Cs2CO3-catalysed lignin degradation. A detailed GC-MS analysis for these samples identified a range of methoxy capped-monomeric degradation products. The scope of this reaction system was further expanded to lignocellulosic biomass such as milled beechwood chips, which notably showed similar product distributions. Based on the correlation of the experimental observations for extracted lignin samples and model compound studies, a mechanistic pathway for the Cs2CO3-catalysed system was suggested. DFT calculations provided reaction pathways for the observed cleavage products.
中文翻译:

碳酸二甲酯中碱催化木质素解聚的机理研究
不断消耗的化石储层刺激了全球对可再生木质素原料作为石油衍生的芳烃的可持续替代品的研究计划。碱催化解聚(BCD)被认为是对工业木质素进行增值的一种经济有效的方法。在该过程中遇到的主要限制因素是所形成的酚产物的再缩合,这导致较低的单体产率。为了减少这些副反应,我们选择了在碳酸二甲酯(DMC)中的碱土金属催化剂,以生产甲基化的苯酚衍生物作为最终产物。在此处,我们首次证明了一种碱促进的解聚工艺,可高收率(52-67 wt%)提供低分子量油,其中所用的碱以真正的催化量使用(催化剂负载量约为5 mol%)。使用碳酸铯和锂在四种不同的木质素样品(1种硫酸盐,3种有机溶剂)上证明了该方法的一般适用性。叔丁醇盐作为催化剂。反应后木质素样品的2D NMR研究表明,两个碱基的主要木质素键均发生了类似的降解。通过定量31 P NMR分析揭示了酚类部分还原的差异。此外,GPC分析表明,对于Cs 2 CO 3而言,向低质量碎片的转变明显-催化的木质素降解。对这些样品进行的详细GC-MS分析确定了一系列甲氧基封端的单体降解产物。该反应系统的范围进一步扩大到木质纤维素生物质,例如碾碎的山毛榉木屑,其表现出相似的产物分布。基于提取的木质素样品的实验观察结果与模型化合物研究的相关性,提出了Cs 2 CO 3催化系统的机理途径。DFT计算提供了观察到的裂解产物的反应途径。
更新日期:2018-01-02
中文翻译:

碳酸二甲酯中碱催化木质素解聚的机理研究
不断消耗的化石储层刺激了全球对可再生木质素原料作为石油衍生的芳烃的可持续替代品的研究计划。碱催化解聚(BCD)被认为是对工业木质素进行增值的一种经济有效的方法。在该过程中遇到的主要限制因素是所形成的酚产物的再缩合,这导致较低的单体产率。为了减少这些副反应,我们选择了在碳酸二甲酯(DMC)中的碱土金属催化剂,以生产甲基化的苯酚衍生物作为最终产物。在此处,我们首次证明了一种碱促进的解聚工艺,可高收率(52-67 wt%)提供低分子量油,其中所用的碱以真正的催化量使用(催化剂负载量约为5 mol%)。使用碳酸铯和锂在四种不同的木质素样品(1种硫酸盐,3种有机溶剂)上证明了该方法的一般适用性。叔丁醇盐作为催化剂。反应后木质素样品的2D NMR研究表明,两个碱基的主要木质素键均发生了类似的降解。通过定量31 P NMR分析揭示了酚类部分还原的差异。此外,GPC分析表明,对于Cs 2 CO 3而言,向低质量碎片的转变明显-催化的木质素降解。对这些样品进行的详细GC-MS分析确定了一系列甲氧基封端的单体降解产物。该反应系统的范围进一步扩大到木质纤维素生物质,例如碾碎的山毛榉木屑,其表现出相似的产物分布。基于提取的木质素样品的实验观察结果与模型化合物研究的相关性,提出了Cs 2 CO 3催化系统的机理途径。DFT计算提供了观察到的裂解产物的反应途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号