当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
One-step rapid synthesis of π-conjugated large oligomers via C–H activation coupling
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7qo00960g Shi-Yong Liu 1, 2, 3, 4, 5 , Di-Gang Wang 5, 6, 7, 8 , Ai-Guo Zhong 5, 6, 7, 8 , He-Rui Wen 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1039/c7qo00960g Shi-Yong Liu 1, 2, 3, 4, 5 , Di-Gang Wang 5, 6, 7, 8 , Ai-Guo Zhong 5, 6, 7, 8 , He-Rui Wen 1, 2, 3, 4
Affiliation
|
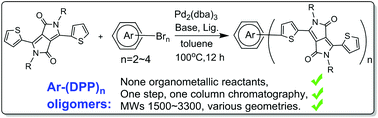
A variety of diketopyrrolopyrrole (DPP)-based linear or branched π-conjugated oligomers, with a core of phenyl, phenyl-DPP, tetraphenylethene, pyrene and spirobifluorene, containing sequentially bis-, tris-, and tetra-DPP with various geometries and molecular weights of several thousands has been effectively accessed by one-step C–H activated coupling followed by a single column chromatographic separation. A further optical property study of all eight synthesized oligomers reveals the correlation between light absorption and the variation of geometries and π-conjugation lengths. This atom- and step-economical, and versatile synthetic strategy will be a powerful tool for accessing π-functional large oligomers, which enriches the library of π-materials and thus facilitates the fundamental structure–property study.
中文翻译:

通过C–H活化偶联一步一步合成π共轭大低聚物
各种基于二酮吡咯并吡咯(DPP)的线性或支化π-共轭低聚物,具有苯基,苯基-DPP,四苯基乙烯,pyr和螺二芴的核心,依次包含具有不同几何结构和分子的双-,三-和四-DPP一步进行C–H活化偶合,然后进行单柱色谱分离,已有效地获得了数千重量。对所有八种合成低聚物的进一步光学性质研究表明,光吸收与几何形状和π共轭长度的变化之间具有相关性。这种原子经济和阶梯经济的多用途合成策略将成为访问π功能大型低聚物的有力工具,它丰富了π材料的库,从而促进了基本结构-性质的研究。
更新日期:2017-11-23
中文翻译:

通过C–H活化偶联一步一步合成π共轭大低聚物
各种基于二酮吡咯并吡咯(DPP)的线性或支化π-共轭低聚物,具有苯基,苯基-DPP,四苯基乙烯,pyr和螺二芴的核心,依次包含具有不同几何结构和分子的双-,三-和四-DPP一步进行C–H活化偶合,然后进行单柱色谱分离,已有效地获得了数千重量。对所有八种合成低聚物的进一步光学性质研究表明,光吸收与几何形状和π共轭长度的变化之间具有相关性。这种原子经济和阶梯经济的多用途合成策略将成为访问π功能大型低聚物的有力工具,它丰富了π材料的库,从而促进了基本结构-性质的研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号