当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How and why a [Bu4P][Im]/CO2 system efficiently catalyzes the hydration of propargylic alcohols to α-hydroxy ketones: electrostatically controlled reactivity
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7cy01646h Xueli Mu 1, 2, 3, 4, 5 , Chengbu Liu 1, 2, 3, 4, 5 , Dongju Zhang 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 5 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7cy01646h Xueli Mu 1, 2, 3, 4, 5 , Chengbu Liu 1, 2, 3, 4, 5 , Dongju Zhang 1, 2, 3, 4, 5
Affiliation
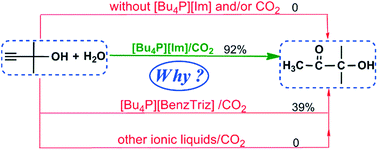
|
Density functional theory calculations have been performed to understand the intriguing experimental observations on the hydration of propargylic alcohols to α-hydroxy ketones catalyzed by task-specific ionic liquids (ILs) and CO2. Focusing on a representative propargylic alcohol, 2-methylbut-3-yn-2-ol, we explored its hydration mechanism and the catalytic reactivities of different ILs towards the reaction in detail. The calculated results show the electrostatically controlled character of the reaction, where the reactivity depends on not only the anion's own nature but also its counterion cation that can regulate and control the anion basicity via electrostatic and H-bonding interactions. The reaction is proposed to proceed via an energetically viable mechanism that features the initial addition of CO2 to the hydroxyl group of the propargylic alcohol with assistance of the IL anion as a proton acceptor. The different catalytic performances of several ILs are attributed to their different proton-accepting capabilities. The best catalytic performance of [Bu4P][Im] is ascribed to its most efficient proton-accepting properties. The theoretical results provide a foundation for exploiting the controlled synthesis of α-hydroxy ketones as well as cyclic carbonates and oxazolidinones from the hydration of propargylic alcohols or propargylic amines.
中文翻译:

[Bu 4 P] [Im] / CO 2系统如何以及为何有效地催化炔丙醇水合为α-羟基酮:静电控制的反应性
已经进行了密度泛函理论计算,以了解关于在特定任务的离子液体(ILs)和CO 2催化下炔丙醇水合为α-羟基酮的有趣实验观察。着眼于代表性的炔丙醇2-甲基丁-3-yn-2-ol,我们详细探讨了其水合机理以及不同IL对反应的催化反应性。计算结果显示了反应的静电控制特性,其中反应性不仅取决于阴离子本身的性质,还取决于其抗衡阳离子,后者可以通过静电和氢键相互作用调节和控制阴离子的碱性。建议该反应通过一种在能量上可行的机制,其特征在于在IL阴离子作为质子受体的辅助下,将CO 2初始添加到炔丙醇的羟基中。几种离子液体的不同催化性能归因于它们不同的质子接受能力。[Bu 4 P] [Im]的最佳催化性能归因于其最有效的质子接受性能。该理论结果为开发由炔丙醇或炔丙胺的水合控制α-羟基酮以及环状碳酸酯和恶唑烷酮的合成提供了基础。
更新日期:2017-11-23
中文翻译:

[Bu 4 P] [Im] / CO 2系统如何以及为何有效地催化炔丙醇水合为α-羟基酮:静电控制的反应性
已经进行了密度泛函理论计算,以了解关于在特定任务的离子液体(ILs)和CO 2催化下炔丙醇水合为α-羟基酮的有趣实验观察。着眼于代表性的炔丙醇2-甲基丁-3-yn-2-ol,我们详细探讨了其水合机理以及不同IL对反应的催化反应性。计算结果显示了反应的静电控制特性,其中反应性不仅取决于阴离子本身的性质,还取决于其抗衡阳离子,后者可以通过静电和氢键相互作用调节和控制阴离子的碱性。建议该反应通过一种在能量上可行的机制,其特征在于在IL阴离子作为质子受体的辅助下,将CO 2初始添加到炔丙醇的羟基中。几种离子液体的不同催化性能归因于它们不同的质子接受能力。[Bu 4 P] [Im]的最佳催化性能归因于其最有效的质子接受性能。该理论结果为开发由炔丙醇或炔丙胺的水合控制α-羟基酮以及环状碳酸酯和恶唑烷酮的合成提供了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号