当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of JTZ-951: A HIF Prolyl Hydroxylase Inhibitor for the Treatment of Renal Anemia
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00404 Yosuke Ogoshi 1 , Takuya Matsui 1 , Ikuo Mitani 1 , Masahiro Yokota 1 , Masakazu Terashita 1 , Dai Motoda 1 , Kazuhito Ueyama 1 , Takahiro Hotta 1 , Takashi Ito 1 , Yasunori Hase 1 , Kenji Fukui 1 , Katsuya Deai 1 , Hiromi Yoshiuchi 1 , Soichiro Ito 1 , Hiroyuki Abe 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00404 Yosuke Ogoshi 1 , Takuya Matsui 1 , Ikuo Mitani 1 , Masahiro Yokota 1 , Masakazu Terashita 1 , Dai Motoda 1 , Kazuhito Ueyama 1 , Takahiro Hotta 1 , Takashi Ito 1 , Yasunori Hase 1 , Kenji Fukui 1 , Katsuya Deai 1 , Hiromi Yoshiuchi 1 , Soichiro Ito 1 , Hiroyuki Abe 1
Affiliation
|
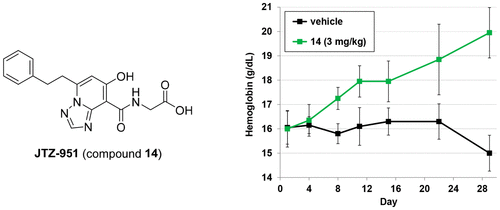
Inhibition of hypoxia inducible factor prolyl hydroxylase (PHD) represents a promising strategy for the discovery of a next generation treatment for renal anemia. We identified several 5,6-fused ring systems as novel scaffolds of the PHD inhibitor on the basis of pharmacophore analysis. In particular, triazolopyridine derivatives showed potent PHD2 inhibitory activities. Examination of the predominance of the triazolopyridines in potency by electrostatic calculations suggested favorable π–π stacking interactions with Tyr310. Lead optimization to improve the efficacy of erythropoietin release in cells and in vivo by improving cell permeability led to the discovery of JTZ-951 (compound 14), with a 5-phenethyl substituent on the triazolopyridine group, which increased hemoglobin levels with daily oral dosing in rats. Compound 14 was rapidly absorbed after oral administration and disappeared shortly thereafter, which could be advantageous in terms of safety. Compound 14 was selected as a clinical candidate.
中文翻译:

JTZ-951的发现:用于治疗肾性贫血的HIF脯氨酰羟化酶抑制剂
缺氧诱导因子脯氨酰羟化酶(PHD)的抑制代表了发现肾脏贫血的下一代治疗方法的一种有前途的策略。我们在药效基团分析的基础上,鉴定了几种5,6-稠环系统为PHD抑制剂的新型支架。特别地,三唑并吡啶衍生物显示出有效的PHD2抑制活性。通过静电计算检查了三唑并吡啶在效能上的优势,表明与Tyr310有良好的π-π堆积相互作用。通过改善细胞通透性来优化细胞和体内促红细胞生成素释放的功效的铅优化导致了JTZ-951的发现(化合物14),在三唑并吡啶基团上具有5-苯乙基取代基,在大鼠中每日口服可增加血红蛋白水平。口服给药后,化合物14被迅速吸收,此后不久就消失了,就安全性而言可能是有利的。选择化合物14作为临床候选物。
更新日期:2017-11-22
中文翻译:

JTZ-951的发现:用于治疗肾性贫血的HIF脯氨酰羟化酶抑制剂
缺氧诱导因子脯氨酰羟化酶(PHD)的抑制代表了发现肾脏贫血的下一代治疗方法的一种有前途的策略。我们在药效基团分析的基础上,鉴定了几种5,6-稠环系统为PHD抑制剂的新型支架。特别地,三唑并吡啶衍生物显示出有效的PHD2抑制活性。通过静电计算检查了三唑并吡啶在效能上的优势,表明与Tyr310有良好的π-π堆积相互作用。通过改善细胞通透性来优化细胞和体内促红细胞生成素释放的功效的铅优化导致了JTZ-951的发现(化合物14),在三唑并吡啶基团上具有5-苯乙基取代基,在大鼠中每日口服可增加血红蛋白水平。口服给药后,化合物14被迅速吸收,此后不久就消失了,就安全性而言可能是有利的。选择化合物14作为临床候选物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号