当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic asymmetric C2-nucleophilic substitutions of C3-substituted indoles with ortho-hydroxybenzyl alcohols
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7qo00649g Jia-Le Wu 1, 2, 3, 4 , Jing-Yi Wang 1, 2, 3, 4 , Ping Wu 1, 2, 3, 4 , Guang-Jian Mei 1, 2, 3, 4 , Feng Shi 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2017-09-11 00:00:00 , DOI: 10.1039/c7qo00649g Jia-Le Wu 1, 2, 3, 4 , Jing-Yi Wang 1, 2, 3, 4 , Ping Wu 1, 2, 3, 4 , Guang-Jian Mei 1, 2, 3, 4 , Feng Shi 1, 2, 3, 4
Affiliation
|
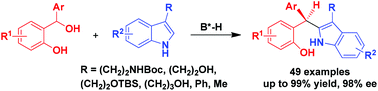
Catalytic asymmetric C2-nucleophilic substitutions of C3-substituted indoles such as tryptophols and tryptamines with ortho-hydroxybenzyl alcohols have been established, leading to the generation of triaryl methane products in generally high yields and excellent enantioselectivities (up to 99% yield, 98% ee). This class of C2-nucleophilic substitutions has wide substrate scope, which is not only applicable to a wide range of ortho-hydroxybenzyl alcohols, but also amenable to various tryptophols, tryptamines and other C3-substituted indoles. In addition, the reaction could be scaled up with maintained yield and enantioselectivity.
中文翻译:

用邻羟基苄醇催化C3取代的吲哚的不对称C2亲核取代
已经建立了C3取代的吲哚(如三苯酚和色胺)的催化不对称C2亲核取代反应,使用邻羟基苄醇,通常以高收率和优异的对映选择性(高达99%,98%ee)生成三芳基甲烷产物。 )。这类C 2-亲核取代具有广泛的底物范围,其不仅适用于广泛的邻-羟基苄醇,而且适用于各种胰多酚,色胺和其他C 3取代的吲哚。另外,可以在保持产率和对映选择性的同时扩大反应规模。
更新日期:2017-11-22
中文翻译:

用邻羟基苄醇催化C3取代的吲哚的不对称C2亲核取代
已经建立了C3取代的吲哚(如三苯酚和色胺)的催化不对称C2亲核取代反应,使用邻羟基苄醇,通常以高收率和优异的对映选择性(高达99%,98%ee)生成三芳基甲烷产物。 )。这类C 2-亲核取代具有广泛的底物范围,其不仅适用于广泛的邻-羟基苄醇,而且适用于各种胰多酚,色胺和其他C 3取代的吲哚。另外,可以在保持产率和对映选择性的同时扩大反应规模。


























 京公网安备 11010802027423号
京公网安备 11010802027423号