当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rate constant for the H˙ + H2O → ˙OH + H2 reaction at elevated temperatures measured by pulse radiolysis
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7cp06010f Y. Muroya 1, 2, 3 , S. Yamashita 3, 4, 5 , P. Lertnaisat 3, 6, 7 , S. Sanguanmith 8, 9, 10 , J. Meesungnoen 8, 9, 10 , J.-P. Jay-Gerin 8, 9, 10 , Y. Katsumura 3, 4, 5, 6, 7
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-11-01 00:00:00 , DOI: 10.1039/c7cp06010f Y. Muroya 1, 2, 3 , S. Yamashita 3, 4, 5 , P. Lertnaisat 3, 6, 7 , S. Sanguanmith 8, 9, 10 , J. Meesungnoen 8, 9, 10 , J.-P. Jay-Gerin 8, 9, 10 , Y. Katsumura 3, 4, 5, 6, 7
Affiliation
|
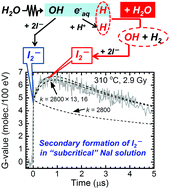
Maintaining the structural integrity of materials in nuclear power plants is an essential issue associated with safe operation. Hydrogen (H2) addition or injection to coolants is a powerful technique that has been widely applied such that the reducing conditions in the coolant water avoid corrosion and stress corrosion cracking (SCC). Because the radiation-induced reaction of ˙OH + H2 → H˙ + H2O plays a crucial role in these systems, the rate constant has been measured at operation temperatures of the reactors (285–300 °C) by pulse radiolysis, generating sufficient data for analysis. The reverse reaction H˙ + H2O → ˙OH + H2 is negligibly slow at ambient temperature; however, it accelerates considerably quickly at elevated temperatures. Although the reverse reaction reduces the effectiveness of H2 addition, reliable rate constants have not yet been measured. In this study, the rate constants have been determined in a temperature range of 250–350 °C by pulse radiolysis in an aqueous I− solution.
中文翻译:

H + + H 2 O→OH + H 2反应的速率常数,在升高的温度下通过脉冲辐解测量
维护核电厂中材料的结构完整性是与安全运行相关的重要问题。向冷却剂中添加或注入氢(H 2)是一项功能强大的技术,已广泛应用,以使冷却剂水中的还原条件避免腐蚀和应力腐蚀开裂(SCC)。由于˙OH+ H 2 →H˙+ H 2 O的辐射诱导反应在这些系统中起关键作用,因此已通过脉冲辐射分解法在反应器的工作温度(285–300°C)下测量了速率常数,生成足够的数据进行分析。逆反应H˙+ H 2 O→˙OH+ H 2在环境温度下缓慢到可以忽略不计;但是,它在高温下会迅速加速。尽管逆反应降低了H 2添加的有效性,但尚未测量出可靠的速率常数。在这项研究中,速率常数已在温度范围250-350℃下,在水性我确定通过脉冲辐-溶液。
更新日期:2017-11-22
中文翻译:

H + + H 2 O→OH + H 2反应的速率常数,在升高的温度下通过脉冲辐解测量
维护核电厂中材料的结构完整性是与安全运行相关的重要问题。向冷却剂中添加或注入氢(H 2)是一项功能强大的技术,已广泛应用,以使冷却剂水中的还原条件避免腐蚀和应力腐蚀开裂(SCC)。由于˙OH+ H 2 →H˙+ H 2 O的辐射诱导反应在这些系统中起关键作用,因此已通过脉冲辐射分解法在反应器的工作温度(285–300°C)下测量了速率常数,生成足够的数据进行分析。逆反应H˙+ H 2 O→˙OH+ H 2在环境温度下缓慢到可以忽略不计;但是,它在高温下会迅速加速。尽管逆反应降低了H 2添加的有效性,但尚未测量出可靠的速率常数。在这项研究中,速率常数已在温度范围250-350℃下,在水性我确定通过脉冲辐-溶液。



























 京公网安备 11010802027423号
京公网安备 11010802027423号