当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiple interaction regulated phase transition behavior of thermo-responsive copolymers containing cationic poly(ionic liquid)s
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1039/c7cp05846b Yingna Zhang 1, 2, 3, 4 , Hui Tang 1, 2, 3, 4 , Peiyi Wu 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2017-10-31 00:00:00 , DOI: 10.1039/c7cp05846b Yingna Zhang 1, 2, 3, 4 , Hui Tang 1, 2, 3, 4 , Peiyi Wu 1, 2, 3, 4
Affiliation
|
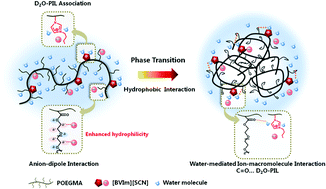
The effect of multiple interactions including anion–macromolecule interaction, water-mediated ion–macromolecule interaction and hydrophobic interaction on the phase transition behaviors of random copolymers P(OEGMA-co-BVIm[X]) comprising oligo(ethylene glycol)methacrylate (OEGMA) and imidazolium-based ionic liquids is investigated in the present study. Temperature-variable 1H NMR and FT-IR investigations demonstrated that the hydration of CH2 side chains in P(OEGMA-co-BVIm[SCN]) was enhanced due to the anion–dipole interaction between a chaotropic anion SCN− and CH2 groups, and dehydration of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups served as the driving force of phase transition. In particular, the formation of C
O groups served as the driving force of phase transition. In particular, the formation of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C
O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.
O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.
中文翻译:

含阳离子聚(离子液体)的热响应共聚物的多重相互作用调节相变行为
阴离子-大分子相互作用,水介导的离子-大分子相互作用和疏水相互作用等多种相互作用对包含低聚(乙二醇)甲基丙烯酸甲酯(OEGMA)的无规共聚物P(OEGMA- co- BVIm [X])的相变行为的影响本研究研究了咪唑类离子液体。温度可变1 H NMR和FT-IR的调查表明,CH的水合2侧链在P(OEGMA-共-BVIm [SCN])中的溶液增强由于离液阴离子SCN之间的阴离子-偶极相互作用-和CH 2团和C的脱水![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) O组是相变的驱动力。特别是,考虑到IL–D 2 O缔合和C O基团的相互作用,在相变过程中P(OEGMA- co -BVIm [SCN])中优选形成C
O组是相变的驱动力。特别是,考虑到IL–D 2 O缔合和C O基团的相互作用,在相变过程中P(OEGMA- co -BVIm [SCN])中优选形成C ![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D 2 O–PIL氢键。这种水介导的离子-大分子相互作用充当聚合物之间的“连接”,导致共聚物逐渐脱水并形成稳定的小尺寸胶束。至于P(OEGMA- co -BVIm [NTf 2
O⋯D 2 O–PIL氢键。这种水介导的离子-大分子相互作用充当聚合物之间的“连接”,导致共聚物逐渐脱水并形成稳定的小尺寸胶束。至于P(OEGMA- co -BVIm [NTf 2![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) ]),加热后将水分子从聚合物链中依次挤出,并通过OEGMA侧链与包裹在聚集物中的IL链段之间的疏水相互作用进行聚合物链的自聚集。
]),加热后将水分子从聚合物链中依次挤出,并通过OEGMA侧链与包裹在聚集物中的IL链段之间的疏水相互作用进行聚合物链的自聚集。
更新日期:2017-11-22
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups served as the driving force of phase transition. In particular, the formation of C
O groups served as the driving force of phase transition. In particular, the formation of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C
O⋯D2O–PIL hydrogen bonds was preferred in P(OEGMA-co-BVIm[SCN]) during the phase transition process considering the interaction of IL–D2O associations and C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.
O groups. This water-mediated ion–macromolecule interaction acted as a “linkage” among polymers, resulting in the gradual dehydration of copolymers and the formation of stable small size micelles. As for P(OEGMA-co-BVIm[NTf2]), water molecules were sequentially squeezed out of the polymer chains upon heating and the self-aggregation of polymer chains was carried out through hydrophobic interaction between OEGMA side chains with IL segments wrapped in the aggregates.
中文翻译:

含阳离子聚(离子液体)的热响应共聚物的多重相互作用调节相变行为
阴离子-大分子相互作用,水介导的离子-大分子相互作用和疏水相互作用等多种相互作用对包含低聚(乙二醇)甲基丙烯酸甲酯(OEGMA)的无规共聚物P(OEGMA- co- BVIm [X])的相变行为的影响本研究研究了咪唑类离子液体。温度可变1 H NMR和FT-IR的调查表明,CH的水合2侧链在P(OEGMA-共-BVIm [SCN])中的溶液增强由于离液阴离子SCN之间的阴离子-偶极相互作用-和CH 2团和C的脱水
![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) O组是相变的驱动力。特别是,考虑到IL–D 2 O缔合和C O基团的相互作用,在相变过程中P(OEGMA- co -BVIm [SCN])中优选形成C
O组是相变的驱动力。特别是,考虑到IL–D 2 O缔合和C O基团的相互作用,在相变过程中P(OEGMA- co -BVIm [SCN])中优选形成C ![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) O⋯D 2 O–PIL氢键。这种水介导的离子-大分子相互作用充当聚合物之间的“连接”,导致共聚物逐渐脱水并形成稳定的小尺寸胶束。至于P(OEGMA- co -BVIm [NTf 2
O⋯D 2 O–PIL氢键。这种水介导的离子-大分子相互作用充当聚合物之间的“连接”,导致共聚物逐渐脱水并形成稳定的小尺寸胶束。至于P(OEGMA- co -BVIm [NTf 2![[双键,长度为m-破折号]](http://www.rsc.org/images/entities/char_e001.gif) ]),加热后将水分子从聚合物链中依次挤出,并通过OEGMA侧链与包裹在聚集物中的IL链段之间的疏水相互作用进行聚合物链的自聚集。
]),加热后将水分子从聚合物链中依次挤出,并通过OEGMA侧链与包裹在聚集物中的IL链段之间的疏水相互作用进行聚合物链的自聚集。



























 京公网安备 11010802027423号
京公网安备 11010802027423号