当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Heterocyclic carbene-catalyzed sulfa-Michael addition of enals
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1039/c7cc07269d Zi-Song Cong 1, 2, 3, 4, 5 , Yang-Guo Li 1, 2, 3, 4, 5 , Guang-Fen Du 1, 2, 3, 4, 5 , Cheng-Zhi Gu 1, 2, 3, 4, 5 , Bin Dai 1, 2, 3, 4, 5 , Lin He 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1039/c7cc07269d Zi-Song Cong 1, 2, 3, 4, 5 , Yang-Guo Li 1, 2, 3, 4, 5 , Guang-Fen Du 1, 2, 3, 4, 5 , Cheng-Zhi Gu 1, 2, 3, 4, 5 , Bin Dai 1, 2, 3, 4, 5 , Lin He 1, 2, 3, 4, 5
Affiliation
|
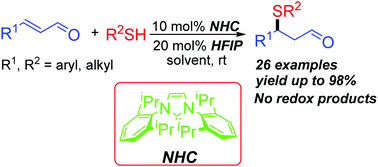
An efficient N-heterocyclic carbene (NHC) catalyzed sulfa-Michael addition (SMA) between enals and thiols has been developed. Under the catalysis of 10 mol% stable free carbene IPr and with 20 mol% hexafluoroisopropanol (HFIP) as an additive, enals react with a variety of thiols to afford the SMA adducts in 54–98% yields. In this process, the free carbene preferentially interacts with thiols through hydrogen-bonding and no NHC-catalyzed extended Umpolung transformations were observed.
中文翻译:

N-杂环卡宾催化的磺胺-迈克尔加成
已经开发了一种有效的N-杂环卡宾(NHC)催化的烯醛和硫醇之间的磺胺-迈克尔加成(SMA)。在10摩尔%稳定的游离卡宾IPr的催化下,并以20摩尔%的六氟异丙醇(HFIP)作为添加剂,使烯醛与各种硫醇反应,以54-98%的产率提供SMA加合物。在此过程中,游离卡宾通过氢键优先与硫醇相互作用,并且未观察到NHC催化的扩展Umpolung转化。
更新日期:2017-11-22
中文翻译:

N-杂环卡宾催化的磺胺-迈克尔加成
已经开发了一种有效的N-杂环卡宾(NHC)催化的烯醛和硫醇之间的磺胺-迈克尔加成(SMA)。在10摩尔%稳定的游离卡宾IPr的催化下,并以20摩尔%的六氟异丙醇(HFIP)作为添加剂,使烯醛与各种硫醇反应,以54-98%的产率提供SMA加合物。在此过程中,游离卡宾通过氢键优先与硫醇相互作用,并且未观察到NHC催化的扩展Umpolung转化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号