当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Amide synthesis via nickel-catalysed reductive aminocarbonylation of aryl halides with nitroarenes
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03950f Chi Wai Cheung 1, 2, 3, 4, 5 , Marten Leendert Ploeger 1, 2, 3, 4, 5 , Xile Hu 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2017-11-06 00:00:00 , DOI: 10.1039/c7sc03950f Chi Wai Cheung 1, 2, 3, 4, 5 , Marten Leendert Ploeger 1, 2, 3, 4, 5 , Xile Hu 1, 2, 3, 4, 5
Affiliation
|
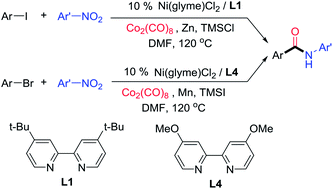
Aminocarbonylation of aryl halides is one of the most useful methods in amide synthesis, but nitroarenes have not been used as a nitrogen source in this method even though they are more economical and accessible than anilines. Reported here is the development of nickel catalysis for the first three-component reactions of aryl halides, Co2(CO)8, and nitroarenes under reductive conditions to produce aryl amides. A wide range of (hetero)aryl iodides and bromides as well as nitro(hetero)arenes are suitable reaction partners, and high functional group compatibility has been achieved. The method might be used for the streamlined synthesis of aryl amides.
中文翻译:

酰胺合成通过芳基卤化物与硝基芳烃镍催化的氨基羰基化还原
芳基卤化物的氨基羰基化是酰胺合成中最有用的方法之一,但是硝基芳烃虽然比苯胺更经济,更易获得,但在该方法中并未用作氮源。此处报道的是在还原条件下,芳基卤化物,Co 2(CO)8和硝基芳烃在头三组分反应中生成镍酰胺的镍催化的进展。广泛的(杂)芳基碘化物和溴化物以及硝基(杂)芳烃是合适的反应伙伴,并且已经实现了高官能团相容性。该方法可用于芳基酰胺的流线型合成。
更新日期:2017-11-22
中文翻译:

酰胺合成通过芳基卤化物与硝基芳烃镍催化的氨基羰基化还原
芳基卤化物的氨基羰基化是酰胺合成中最有用的方法之一,但是硝基芳烃虽然比苯胺更经济,更易获得,但在该方法中并未用作氮源。此处报道的是在还原条件下,芳基卤化物,Co 2(CO)8和硝基芳烃在头三组分反应中生成镍酰胺的镍催化的进展。广泛的(杂)芳基碘化物和溴化物以及硝基(杂)芳烃是合适的反应伙伴,并且已经实现了高官能团相容性。该方法可用于芳基酰胺的流线型合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号