当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Cocatalytic Electron‐Transfer Cascade Site‐Selectively Placed on TiO2 Nanotubes Yields Enhanced Photocatalytic H2 Evolution
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2017-11-22 , DOI: 10.1002/adfm.201704259 Marco Altomare 1 , Nhat Truong Nguyen 1 , Seyedsina Hejazi 1 , Patrik Schmuki 1, 2
Advanced Functional Materials ( IF 19.0 ) Pub Date : 2017-11-22 , DOI: 10.1002/adfm.201704259 Marco Altomare 1 , Nhat Truong Nguyen 1 , Seyedsina Hejazi 1 , Patrik Schmuki 1, 2
Affiliation
|
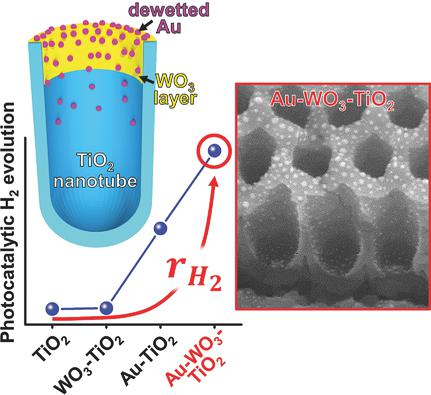
Separation and transfer of photogenerated charge carriers are key elements in designing photocatalysts. TiO2 in numerous geometries has been for many years the most studied photocatalyst. To overcome kinetic limitations and achieve swift charge transfer, TiO2 has been widely investigated with cocatalysts that are commonly randomly placed nanoparticles on a TiO2 surface. The poor control over cocatalyst placement in powder technology approaches can drastically hamper the photocatalytic efficiencies. Here in contrast it is shown that the site‐selective placement of suitable charge‐separation and charge‐transfer cocatalysts on a defined TiO2 nanotube morphology can provide an enhancement of the photocatalytic reactivity. A TiO2–WO3–Au electron‐transfer cascade photocatalyst is designed with nanoscale precision for H2 production on TiO2 nanotube arrays. Key aspects in the construction are the placement of the WO3/Au element at the nanotube top by site‐selective deposition and self‐ordered thermal dewetting of Au. In the ideal configuration, WO3 acts as a buffer layer for TiO2 conduction band electrons, allowing for their efficient transfer to the Au nanoparticles and then to a suitable environment for H2 generation, while TiO2 holes due to intrinsic upward band bending in the nanotube walls and short diffusion length undergo a facilitated transfer to the electrolyte where oxidation of hole‐scavenger molecules takes place. These photocatalytic structures can achieve H2 generation rates significantly higher than any individual cocatalyst–TiO2 combination, including a classic noble metal–TiO2 configuration.
中文翻译:

选择性地放置在TiO2纳米管上的共催化电子转移级联产生增强的光催化H2演化
光生载流子的分离和转移是设计光催化剂的关键要素。多年来,在许多几何形状中的TiO 2一直是研究最多的光催化剂。为了克服动力学限制并实现快速的电荷转移,已经广泛地使用助催化剂对TiO 2进行了研究,助催化剂通常是将纳米颗粒随机放置在TiO 2表面上。在粉末技术方法中对助催化剂放置的控制不力会严重阻碍光催化效率。相比之下,这表明在定义的TiO 2纳米管形态上适当的电荷分离和电荷转移助催化剂的位置选择性放置可以增强光催化反应活性。钛白粉2 – WO 3 –Au电子转移级联光催化剂以纳米级精度设计,用于在TiO 2纳米管阵列上生产H 2。该结构的关键方面是通过定点沉积和Au的自序热去湿将WO 3 / Au元素放置在纳米管顶部。在理想的配置中,WO 3充当TiO 2导带电子的缓冲层,从而使其能够有效转移到Au纳米颗粒,然后转移到合适的环境中以产生H 2,而TiO 2由于纳米管壁上固有的向上能带弯曲和扩散长度短而导致的空穴容易转移到电解质中,在该电解质中发生空穴清除剂分子的氧化。这些光催化结构可实现的H 2生成速率显着高于任何单独的助催化剂– TiO 2组合,包括经典的贵金属– TiO 2构型。
更新日期:2017-11-22
中文翻译:

选择性地放置在TiO2纳米管上的共催化电子转移级联产生增强的光催化H2演化
光生载流子的分离和转移是设计光催化剂的关键要素。多年来,在许多几何形状中的TiO 2一直是研究最多的光催化剂。为了克服动力学限制并实现快速的电荷转移,已经广泛地使用助催化剂对TiO 2进行了研究,助催化剂通常是将纳米颗粒随机放置在TiO 2表面上。在粉末技术方法中对助催化剂放置的控制不力会严重阻碍光催化效率。相比之下,这表明在定义的TiO 2纳米管形态上适当的电荷分离和电荷转移助催化剂的位置选择性放置可以增强光催化反应活性。钛白粉2 – WO 3 –Au电子转移级联光催化剂以纳米级精度设计,用于在TiO 2纳米管阵列上生产H 2。该结构的关键方面是通过定点沉积和Au的自序热去湿将WO 3 / Au元素放置在纳米管顶部。在理想的配置中,WO 3充当TiO 2导带电子的缓冲层,从而使其能够有效转移到Au纳米颗粒,然后转移到合适的环境中以产生H 2,而TiO 2由于纳米管壁上固有的向上能带弯曲和扩散长度短而导致的空穴容易转移到电解质中,在该电解质中发生空穴清除剂分子的氧化。这些光催化结构可实现的H 2生成速率显着高于任何单独的助催化剂– TiO 2组合,包括经典的贵金属– TiO 2构型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号