当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ozone Decomposition on Kaolinite as a Function of Monoterpene Exposure and Relative Humidity
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00107 Zoe L. Coates Fuentes 1 , Theresa M. Kucinski 1 , Ryan Z. Hinrichs 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2017-12-12 00:00:00 , DOI: 10.1021/acsearthspacechem.7b00107 Zoe L. Coates Fuentes 1 , Theresa M. Kucinski 1 , Ryan Z. Hinrichs 1
Affiliation
|
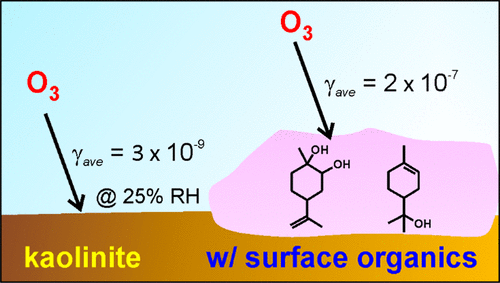
Atmospheric processing of mineral aerosol by trace gases results in the formation of surface-adsorbed products that have the capacity to alter the chemical and physical properties of these airborne particulates. To investigate one potential impact of aerosol processing by biogenic volatile organic compounds (BVOCs), we investigated the heterogeneous decomposition of ozone on pure and monoterpene-processed kaolinite. We used a laminar flow reactor to measure O3 reactive uptake coefficients on kaolinite-coated tubes as a function of relative humidity, O3 concentration, and pre-exposure to gaseous limonene and α-pinene. At 26% RH, kaolinite has a near equivalent of a monolayer of adsorbed water, and the ozone steady-state uptake coefficient was γav = 2.9 × 10–9 assuming the BET surface area. Pre-exposing kaolinite to limonene and α-pinene increased O3 uptake coefficients by nearly 2 orders of magnitude to 2.1 × 10–7 and 2.5 × 10–7, respectively. At all humidities studied (10–50% RH), O3 uptake was at least 1 order of magnitude higher for monoterpene-processed kaolinite compared to that of pure kaolinite. This dramatic increase in O3 reactivity is attributed to surface-adsorbed organics, namely limonenediol and α-terpineol, which contain alkene functionalities susceptible to ozonolysis. Increasing relative humidity decreased O3 uptake for monoterpene-processed kaolinite consistent with competitive adsorption of water resulting in lower organic surface concentrations. These results demonstrate the significant impact adsorbed organics can have on O3 uptake coefficients on mineral aerosol, which should be accounted for in atmospheric modeling studies.
中文翻译:

高岭石上臭氧的分解与单萜暴露和相对湿度的关系
微量气体在大气中处理矿物气溶胶会导致形成表面吸附的产品,这些产品具有改变这些空气传播微粒的化学和物理特性的能力。为了研究生物挥发性有机化合物(BVOC)对气溶胶处理的潜在影响,我们研究了臭氧在纯和单萜处理的高岭石上的异质分解。我们使用层流反应器来测量高岭石涂层管上的O 3反应吸收系数,该系数是相对湿度,O 3浓度以及气态to烯和α-pine烯的预暴露的函数。在相对湿度26%时,高岭石的吸附量几乎等于单层吸附水,臭氧的稳态吸收系数为γav = 2.9×10–9假定为BET表面积。高岭石与柠檬烯和α-pine烯的预暴露使O 3吸收系数分别提高了近2个数量级,分别为2.1×10 –7和2.5×10 –7。在所有研究的湿度(相对湿度10–50%)下,单萜处理的高岭石的O 3吸收量比纯高岭石的O 3吸收量至少高1个数量级。O 3反应性的急剧增加归因于表面吸附的有机物,即柠檬烯二醇和α-萜品醇,它们含有易于臭氧分解的烯烃官能团。相对湿度增加,O 3降低单萜处理的高岭石的吸收与水的竞争性吸附一致,从而导致较低的有机表面浓度。这些结果表明,吸附的有机物可能会对矿物气溶胶的O 3吸收系数产生重大影响,这应该在大气模拟研究中加以考虑。
更新日期:2017-12-12
中文翻译:

高岭石上臭氧的分解与单萜暴露和相对湿度的关系
微量气体在大气中处理矿物气溶胶会导致形成表面吸附的产品,这些产品具有改变这些空气传播微粒的化学和物理特性的能力。为了研究生物挥发性有机化合物(BVOC)对气溶胶处理的潜在影响,我们研究了臭氧在纯和单萜处理的高岭石上的异质分解。我们使用层流反应器来测量高岭石涂层管上的O 3反应吸收系数,该系数是相对湿度,O 3浓度以及气态to烯和α-pine烯的预暴露的函数。在相对湿度26%时,高岭石的吸附量几乎等于单层吸附水,臭氧的稳态吸收系数为γav = 2.9×10–9假定为BET表面积。高岭石与柠檬烯和α-pine烯的预暴露使O 3吸收系数分别提高了近2个数量级,分别为2.1×10 –7和2.5×10 –7。在所有研究的湿度(相对湿度10–50%)下,单萜处理的高岭石的O 3吸收量比纯高岭石的O 3吸收量至少高1个数量级。O 3反应性的急剧增加归因于表面吸附的有机物,即柠檬烯二醇和α-萜品醇,它们含有易于臭氧分解的烯烃官能团。相对湿度增加,O 3降低单萜处理的高岭石的吸收与水的竞争性吸附一致,从而导致较低的有机表面浓度。这些结果表明,吸附的有机物可能会对矿物气溶胶的O 3吸收系数产生重大影响,这应该在大气模拟研究中加以考虑。


























 京公网安备 11010802027423号
京公网安备 11010802027423号