当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Bivalent 5-HT2A Receptor Antagonists Exhibit High Affinity and Potency in Vitro and Efficacy in Vivo
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00309 Claudia A. Soto , Matthew J. Shashack , Robert G. Fox , Marcy J. Bubar , Kenner C. Rice 1 , Cheryl S. Watson , Kathryn A. Cunningham , Scott R. Gilbertson 2 , Noelle C. Anastasio
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acschemneuro.7b00309 Claudia A. Soto , Matthew J. Shashack , Robert G. Fox , Marcy J. Bubar , Kenner C. Rice 1 , Cheryl S. Watson , Kathryn A. Cunningham , Scott R. Gilbertson 2 , Noelle C. Anastasio
Affiliation
|
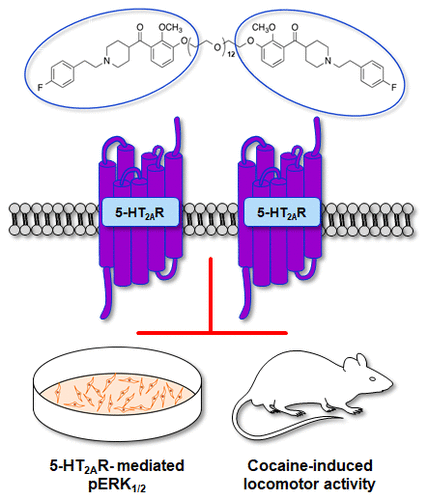
The 5-HT2A receptor (5-HT2AR) plays an important role in various neuropsychiatric disorders, including substance use disorder and schizophrenia. Homodimerization of this receptor has been suggested, but tools that allow direct assessment of the relevance of the 5-HT2AR:5-HT2AR homodimer in these disorders are necessary. We chemically modified the selective 5-HT2AR antagonist M100907 to synthesize a series of homobivalent ligands connected by ethylene glycol linkers of varying lengths that may be useful tools for probing 5-HT2AR:5-HT2AR homodimer function. We tested these molecules for 5-HT2AR antagonist activity in a cell line stably expressing the functional 5-HT2AR and quantified a downstream signaling target, activation (phosphorylation) of extracellular regulated kinases 1/2 (ERK1/2), in comparison to in vivo efficacy of altering spontaneous or cocaine-evoked locomotor activity in rats. All of the synthetic compounds inhibited 5-HT-mediated phosphorylation of ERK1/2 in the cellular signaling assay; the potency of the bivalent ligands varied as a function of linker length, with the intermediate linker lengths being the most potent. The Ki values for the binding of bivalent ligands to 5-HT2AR were only slightly lower than the values for the parent (+)-M100907 compound, but significant selectivity for 5-HT2AR over 5-HT2BR or 5-HT2CR binding was retained. In addition, the 11-atom-linked bivalent 5-HT2AR antagonist (2 mg/kg, intraperitoneally) demonstrated efficacy on par with that of (+)-M100907 in inhibiting cocaine-evoked hyperactivity. As we develop further strategies for ligand-evoked receptor assembly and analyses of diverse signaling and functional roles, these novel homobivalent 5-HT2AR antagonist ligands will serve as useful in vitro and in vivo probes of 5-HT2AR structure and function.
中文翻译:

新型的二价5-HT 2A受体拮抗剂表现出高亲和力和体外效能,体内功效
5-HT 2A受体(5-HT 2A R)在各种神经精神疾病中起重要作用,包括药物滥用和精神分裂症。已经提出了该受体的均二聚化,但是需要用于直接评估5-HT 2A R:5-HT 2A R同型二聚体在这些疾病中的相关性的工具。我们化学修饰了选择性5-HT 2A R拮抗剂M100907,以合成一系列不同长度的乙二醇接头连接的同型二价配体,这可能是探测5-HT 2A R:5-HT 2A R同型二聚体功能的有用工具。我们测试了这些分子的5-HT 2A与体内自发改变的功效相比,R拮抗剂在稳定表达功能性5-HT 2A R的细胞系中的活性并量化了下游信号传导靶标,即细胞外调节激酶1/2(ERK 1/2)的激活(磷酸化)或可卡因诱发的大鼠运动能力。在细胞信号分析中,所有合成化合物均抑制5-HT介导的ERK 1/2磷酸化。二价配体的效力随接头长度的变化而变化,中间接头长度最有效。二价配体与5-HT 2A结合的K i值R仅略低于母体(+)-M100907化合物的值,但保留了对5-HT 2A R相对于5-HT 2B R或5-HT 2C R结合的显着选择性。此外,11原子连接的二价5-HT 2A R拮抗剂(2 mg / kg,腹膜内)在抑制可卡因引起的机能亢进方面具有与(+)-M100907相当的功效。随着我们为配体诱发的受体装配开发进一步的策略并分析各种信号传导和功能作用,这些新颖的同型二价5-HT 2A R拮抗剂配体将作为有用的5-HT 2A R结构和功能的体外和体内探针。
更新日期:2017-11-22
中文翻译:

新型的二价5-HT 2A受体拮抗剂表现出高亲和力和体外效能,体内功效
5-HT 2A受体(5-HT 2A R)在各种神经精神疾病中起重要作用,包括药物滥用和精神分裂症。已经提出了该受体的均二聚化,但是需要用于直接评估5-HT 2A R:5-HT 2A R同型二聚体在这些疾病中的相关性的工具。我们化学修饰了选择性5-HT 2A R拮抗剂M100907,以合成一系列不同长度的乙二醇接头连接的同型二价配体,这可能是探测5-HT 2A R:5-HT 2A R同型二聚体功能的有用工具。我们测试了这些分子的5-HT 2A与体内自发改变的功效相比,R拮抗剂在稳定表达功能性5-HT 2A R的细胞系中的活性并量化了下游信号传导靶标,即细胞外调节激酶1/2(ERK 1/2)的激活(磷酸化)或可卡因诱发的大鼠运动能力。在细胞信号分析中,所有合成化合物均抑制5-HT介导的ERK 1/2磷酸化。二价配体的效力随接头长度的变化而变化,中间接头长度最有效。二价配体与5-HT 2A结合的K i值R仅略低于母体(+)-M100907化合物的值,但保留了对5-HT 2A R相对于5-HT 2B R或5-HT 2C R结合的显着选择性。此外,11原子连接的二价5-HT 2A R拮抗剂(2 mg / kg,腹膜内)在抑制可卡因引起的机能亢进方面具有与(+)-M100907相当的功效。随着我们为配体诱发的受体装配开发进一步的策略并分析各种信号传导和功能作用,这些新颖的同型二价5-HT 2A R拮抗剂配体将作为有用的5-HT 2A R结构和功能的体外和体内探针。


























 京公网安备 11010802027423号
京公网安备 11010802027423号