当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Constant pH Accelerated Molecular Dynamics Investigation of the pH Regulation Mechanism of Dinoflagellate Luciferase
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b00873 Patrick H. Donnan 1 , Phong D. Ngo 1 , Steven O. Mansoorabadi 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b00873 Patrick H. Donnan 1 , Phong D. Ngo 1 , Steven O. Mansoorabadi 1
Affiliation
|
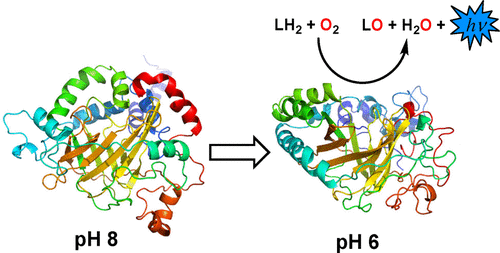
The bioluminescence reaction in dinoflagellates involves the oxidation of an open-chain tetrapyrrole by the enzyme dinoflagellate luciferase (LCF). The activity of LCF is tightly regulated by pH, where the enzyme is essentially inactive at pH ∼8 and optimally active at pH ∼6. Little is known about the mechanism of LCF or the structure of the active form of the enzyme, although it has been proposed that several intramolecularly conserved histidine residues in the N-terminal region are important for the pH regulation mechanism. Here, constant pH accelerated molecular dynamics was employed to gain insight into the conformational activation of LCF induced by acidification.
中文翻译:

鞭毛萤光素荧光素酶pH调节机制的恒定pH加速分子动力学研究
鞭毛鞭毛虫的生物发光反应涉及双鞭毛鞭毛虫萤光素酶(LCF)氧化开链四吡咯。LCF的活性受pH值严格控制,其中该酶在pH约8时基本上是无活性的,而在pH约6时则是最佳活性的。关于LCF的机制或酶的活性形式的结构知之甚少,尽管已经提出在N端区域中的几个分子内保守的组氨酸残基对于pH调节机制很重要。在这里,恒定的pH加速分子动力学被用来深入了解由酸化诱导的LCF的构象活化。
更新日期:2017-11-22
中文翻译:

鞭毛萤光素荧光素酶pH调节机制的恒定pH加速分子动力学研究
鞭毛鞭毛虫的生物发光反应涉及双鞭毛鞭毛虫萤光素酶(LCF)氧化开链四吡咯。LCF的活性受pH值严格控制,其中该酶在pH约8时基本上是无活性的,而在pH约6时则是最佳活性的。关于LCF的机制或酶的活性形式的结构知之甚少,尽管已经提出在N端区域中的几个分子内保守的组氨酸残基对于pH调节机制很重要。在这里,恒定的pH加速分子动力学被用来深入了解由酸化诱导的LCF的构象活化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号