当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulatory Mechanism of Mycobacterium tuberculosis Phosphoserine Phosphatase SerB2
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b01082 Gregory A. Grant 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.biochem.7b01082 Gregory A. Grant 1
Affiliation
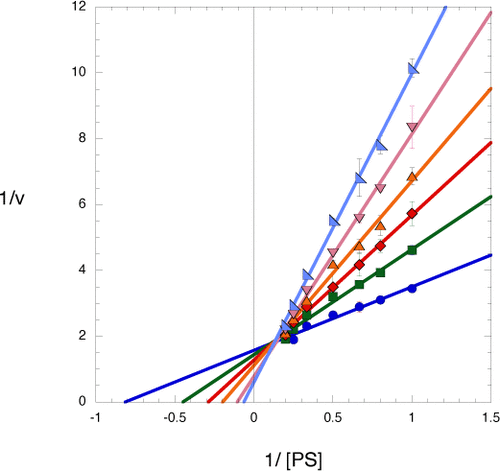
|
Almost all organisms contain the same biosynthetic pathway for the synthesis of l-serine from the glycolytic intermediate, d-3-phosphoglycerate. However, regulation of this pathway varies from organism to organism. Many organisms control the activity of the first enzyme in the pathway, d-3-phosphoglycerate dehydrogenase (PGDH), by feedback inhibition through the interaction of l-serine with the ACT domains within the enzyme. The last enzyme in the pathway, phosphoserine phosphatase (PSP), has also been reported to be inhibited by l-serine. The high degree of sequence homology between Mycobacterium tuberculosis PSP (mtPSP) and Mycobacterium avium PSP (maPSP), which has recently been shown to contain ACT domains, suggested that the mtPSP also contained ACT domains. This raised the question of whether the ACT domains in mtPSP played a functional role similar to that of the ACT domains in PGDH. This investigation reveals that l-serine allosterically inhibits mtPSP by a mechanism of partial competitive inhibition by binding to the ACT domains. Therefore, in mtPSP, l-serine is an allosteric feedback inhibitor that acts by decreasing the affinity of the substrate for the enzyme. mtPGDH is also feedback inhibited by l-serine, but only in the presence of millimolar concentrations of phosphate. Therefore, the inhibition of mtPSP by l-serine would act as a secondary control point for the regulation of the l-serine biosynthetic pathway under physiological conditions where the level of phosphate would be below that needed for l-serine feedback inhibition of mtPGDH.
中文翻译:

结核分枝杆菌磷酸丝氨酸磷酸酶SerB2的调控机制
几乎所有生物体都具有相同的生物合成途径,用于从糖酵解中间体d -3-磷酸甘油酸合成1-丝氨酸。但是,该途径的调节因生物体而异。许多生物通过1-丝氨酸与酶中ACT域的相互作用进行反馈抑制,从而控制该途径中第一个酶d -3-磷酸甘油酸脱氢酶(PGDH)的活性。还已经报道了该途径中的最后一种酶磷酸丝氨酸磷酸酶(PSP)被1-丝氨酸抑制。之间的高度序列同源性的结核分枝杆菌PSP(公吨PSP)和最近已显示鸟分枝杆菌PSP(ma PSP)包含ACT域,这表明mt PSP也包含ACT域。这就提出了一个问题,即mt PSP中的ACT域是否起着与PGDH中的ACT域相似的作用。该研究揭示了1-丝氨酸通过结合至ACT结构域的部分竞争性抑制的机制而变构地抑制mt PSP。因此,在mt PSP中,1-丝氨酸是一种变构反馈抑制剂,其通过降低底物对酶的亲和力起作用。mt PGDH也受到l抑制的反馈-丝氨酸,但仅在浓度为毫摩尔的磷酸盐存在下。因此,抑制MT PSP由升丝氨酸将充当用于调节次级控制点升的生理条件下-丝氨酸生物合成途径,其中磷酸盐的水平将低于所需的升的丝氨酸反馈抑制公吨PGDH 。
更新日期:2017-11-22
中文翻译:

结核分枝杆菌磷酸丝氨酸磷酸酶SerB2的调控机制
几乎所有生物体都具有相同的生物合成途径,用于从糖酵解中间体d -3-磷酸甘油酸合成1-丝氨酸。但是,该途径的调节因生物体而异。许多生物通过1-丝氨酸与酶中ACT域的相互作用进行反馈抑制,从而控制该途径中第一个酶d -3-磷酸甘油酸脱氢酶(PGDH)的活性。还已经报道了该途径中的最后一种酶磷酸丝氨酸磷酸酶(PSP)被1-丝氨酸抑制。之间的高度序列同源性的结核分枝杆菌PSP(公吨PSP)和最近已显示鸟分枝杆菌PSP(ma PSP)包含ACT域,这表明mt PSP也包含ACT域。这就提出了一个问题,即mt PSP中的ACT域是否起着与PGDH中的ACT域相似的作用。该研究揭示了1-丝氨酸通过结合至ACT结构域的部分竞争性抑制的机制而变构地抑制mt PSP。因此,在mt PSP中,1-丝氨酸是一种变构反馈抑制剂,其通过降低底物对酶的亲和力起作用。mt PGDH也受到l抑制的反馈-丝氨酸,但仅在浓度为毫摩尔的磷酸盐存在下。因此,抑制MT PSP由升丝氨酸将充当用于调节次级控制点升的生理条件下-丝氨酸生物合成途径,其中磷酸盐的水平将低于所需的升的丝氨酸反馈抑制公吨PGDH 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号