当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Combined TD-DFT-SOS-CIS(D) Study of BOPHY Derivatives with Potential Application in Biosensing
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b09698 Miguel Ponce-Vargas 1, 2 , Cloé Azarias 3 , Denis Jacquemin 3, 4 , Boris Le Guennic 2
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b09698 Miguel Ponce-Vargas 1, 2 , Cloé Azarias 3 , Denis Jacquemin 3, 4 , Boris Le Guennic 2
Affiliation
|
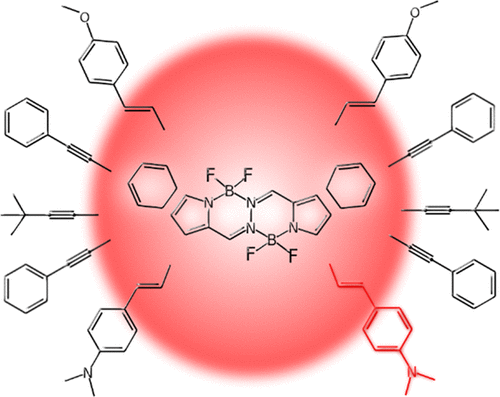
A set of 13 bis(difluoroboron)-1,2-bis((pyrrol-2-yl)methylene)hydrazine (BOPHY) dyes is studied through a hybrid time-dependent density functional theory (TD-DFT)-scaled opposite spin-configuration interaction singles with a double correction [SOS-CIS(D)] approach accounting for solvent effects, to shed light onto the structure–property relationships of these recently developed chromophores. In the first step, we calculate the absorption–fluorescence crossing points with refined TD-DFT models considering the influences of both vibrational and solvent contributions. We found that the systematic overestimation of the 0-0 energies is effectively reduced by combining polarizable continuum model-TD-DFT with a scaled opposite spin-configuration interaction singles with a double correction [SOS-CIS(D)]. Next, for a representative system, the vibrationally resolved spectrum within the harmonic approximation is computed on the basis of TD-DFT vibrational signatures and an excellent match with experiment is found. Finally, the influence of different lateral groups on the spectroscopic properties is rationalized by investigating charge transfer parameters and examining electronic density difference maps. It is found that one can tune the position of the absorption/emission maxima by a judicious choice of the lateral substituents or by using π-extended segments. The largest absorption and emission wavelengths as well as the largest Stokes shifts are obtained for BOPHYs containing strong electron-donor dimethylaminophenyl groups attached to the α-positions of the pyrrole units through vinyl linkers, making these chromophores promising candidates for bioluminescence applications.
中文翻译:

结合TD-DFT-SOS-CIS(D)研究BOPHY衍生物及其在生物传感中的潜在应用
通过混合时变密度泛函理论(TD-DFT)标定了相反的自旋结构,研究了13种双(二氟硼)-1,2-双((吡咯-2-基)亚甲基)肼(BOPHY)染料。构型相互作用单峰采用双校正[SOS-CIS(D)]方法解决了溶剂的影响,从而阐明了这些最近开发的生色团的结构与性质之间的关系。第一步,考虑到振动和溶剂贡献的影响,我们使用精确的TD-DFT模型计算吸收-荧光交叉点。我们发现,通过将可极化的连续谱模型-TD-DFT与带有双校正的比例相反的自旋配置相互作用单相组合,可以有效地减少0-0能量的系统过高估计[SOS-CIS(D)]。接下来,对于一个代表系统,在TD-DFT振动特征的基础上,计算了谐波近似内的振动分辨谱,发现与实验具有很好的匹配性。最后,通过研究电荷转移参数并检查电子密度差图,可以合理地考虑不同侧基对光谱性质的影响。已经发现,可以通过明智地选择侧向取代基或通过使用π-延伸的链段来调节吸收/发射最大值的位置。对于含有通过乙烯基连接基连接到吡咯单元的α位置的强电子给体二甲基氨基苯基的BOPHY,可以获得最大的吸收和发射波长以及最大的斯托克斯位移,这使这些生色团有望成为生物发光应用的候选物。
更新日期:2017-11-22
中文翻译:

结合TD-DFT-SOS-CIS(D)研究BOPHY衍生物及其在生物传感中的潜在应用
通过混合时变密度泛函理论(TD-DFT)标定了相反的自旋结构,研究了13种双(二氟硼)-1,2-双((吡咯-2-基)亚甲基)肼(BOPHY)染料。构型相互作用单峰采用双校正[SOS-CIS(D)]方法解决了溶剂的影响,从而阐明了这些最近开发的生色团的结构与性质之间的关系。第一步,考虑到振动和溶剂贡献的影响,我们使用精确的TD-DFT模型计算吸收-荧光交叉点。我们发现,通过将可极化的连续谱模型-TD-DFT与带有双校正的比例相反的自旋配置相互作用单相组合,可以有效地减少0-0能量的系统过高估计[SOS-CIS(D)]。接下来,对于一个代表系统,在TD-DFT振动特征的基础上,计算了谐波近似内的振动分辨谱,发现与实验具有很好的匹配性。最后,通过研究电荷转移参数并检查电子密度差图,可以合理地考虑不同侧基对光谱性质的影响。已经发现,可以通过明智地选择侧向取代基或通过使用π-延伸的链段来调节吸收/发射最大值的位置。对于含有通过乙烯基连接基连接到吡咯单元的α位置的强电子给体二甲基氨基苯基的BOPHY,可以获得最大的吸收和发射波长以及最大的斯托克斯位移,这使这些生色团有望成为生物发光应用的候选物。

























 京公网安备 11010802027423号
京公网安备 11010802027423号