当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity
ACS Central Science ( IF 18.2 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acscentsci.7b00224 Frederick M. Tomlin 1 , Ulla I. M. Gerling-Driessen 1 , Yi-Chang Liu 1 , Ryan A. Flynn 1 , Janakiram R. Vangala 2 , Christian S. Lentz 3 , Sandra Clauder-Muenster 4 , Petra Jakob 4 , William F. Mueller 4 , Diana Ordoñez-Rueda 4 , Malte Paulsen 4 , Naoko Matsui 5 , Deirdre Foley 5 , Agnes Rafalko 5 , Tadashi Suzuki 6 , Matthew Bogyo 3, 7 , Lars M. Steinmetz 4, 8 , Senthil K. Radhakrishnan 2 , Carolyn R. Bertozzi 1, 9
ACS Central Science ( IF 18.2 ) Pub Date : 2017-10-25 00:00:00 , DOI: 10.1021/acscentsci.7b00224 Frederick M. Tomlin 1 , Ulla I. M. Gerling-Driessen 1 , Yi-Chang Liu 1 , Ryan A. Flynn 1 , Janakiram R. Vangala 2 , Christian S. Lentz 3 , Sandra Clauder-Muenster 4 , Petra Jakob 4 , William F. Mueller 4 , Diana Ordoñez-Rueda 4 , Malte Paulsen 4 , Naoko Matsui 5 , Deirdre Foley 5 , Agnes Rafalko 5 , Tadashi Suzuki 6 , Matthew Bogyo 3, 7 , Lars M. Steinmetz 4, 8 , Senthil K. Radhakrishnan 2 , Carolyn R. Bertozzi 1, 9
Affiliation
|
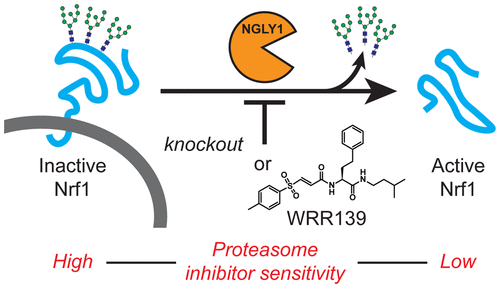
Proteasome inhibitors are used to treat blood cancers such as multiple myeloma (MM) and mantle cell lymphoma. The efficacy of these drugs is frequently undermined by acquired resistance. One mechanism of proteasome inhibitor resistance may involve the transcription factor Nuclear Factor, Erythroid 2 Like 1 (NFE2L1, also referred to as Nrf1), which responds to proteasome insufficiency or pharmacological inhibition by upregulating proteasome subunit gene expression. This “bounce-back” response is achieved through a unique mechanism. Nrf1 is constitutively translocated into the ER lumen, N-glycosylated, and then targeted for proteasomal degradation via the ER-associated degradation (ERAD) pathway. Proteasome inhibition leads to accumulation of cytosolic Nrf1, which is then processed to form the active transcription factor. Here we show that the cytosolic enzyme N-glycanase 1 (NGLY1, the human PNGase) is essential for Nrf1 activation in response to proteasome inhibition. Chemical or genetic disruption of NGLY1 activity results in the accumulation of misprocessed Nrf1 that is largely excluded from the nucleus. Under these conditions, Nrf1 is inactive in regulating proteasome subunit gene expression in response to proteasome inhibition. Through a small molecule screen, we identified a cell-active NGLY1 inhibitor that disrupts the processing and function of Nrf1. The compound potentiates the cytotoxicity of carfilzomib, a clinically used proteasome inhibitor, against MM and T cell-derived acute lymphoblastic leukemia (T-ALL) cell lines. Thus, NGLY1 inhibition prevents Nrf1 activation and represents a new therapeutic approach for cancers that depend on proteasome homeostasis.
中文翻译:

NGLY1的抑制作用使转录因子Nrf1失活并增强蛋白酶体抑制剂的细胞毒性。
蛋白酶体抑制剂用于治疗血液癌症,例如多发性骨髓瘤(MM)和套细胞淋巴瘤。这些药物的功效经常被获得性耐药性破坏。蛋白酶体抑制剂抗性的一种机制可能涉及转录因子核因子,类红细胞2样1(NFE2L1,也称为Nrf1),其通过上调蛋白酶体亚基基因表达来响应蛋白酶体功能不全或药理学抑制。这种“反弹”响应是通过独特的机制实现的。Nrf1组成型易位到ER内腔,N-糖基化,然后通过ER相关降解(ERAD)途径靶向进行蛋白酶体降解。蛋白酶体抑制导致胞浆Nrf1积累,然后将其加工形成活性转录因子。在这里,我们显示了胞质酶N-聚糖酶1(NGLY1,人PNGase)对于Nrf1激活对蛋白酶体抑制起着至关重要的作用。NGLY1活性的化学或遗传破坏会导致错误处理的Nrf1的积累,而Nrf1则在很大程度上被排除在细胞核之外。在这些条件下,Nrf1在调节蛋白酶体亚基时不响应蛋白酶体的抑制作用。通过小分子筛选,我们确定了一种破坏Nrf1加工和功能的具有细胞活性的NGLY1抑制剂。该化合物可增强卡非佐米(一种临床上使用的蛋白酶体抑制剂)对MM和T细胞衍生的急性淋巴细胞白血病(T-ALL)细胞系的细胞毒性。因此,
更新日期:2017-11-22
中文翻译:

NGLY1的抑制作用使转录因子Nrf1失活并增强蛋白酶体抑制剂的细胞毒性。
蛋白酶体抑制剂用于治疗血液癌症,例如多发性骨髓瘤(MM)和套细胞淋巴瘤。这些药物的功效经常被获得性耐药性破坏。蛋白酶体抑制剂抗性的一种机制可能涉及转录因子核因子,类红细胞2样1(NFE2L1,也称为Nrf1),其通过上调蛋白酶体亚基基因表达来响应蛋白酶体功能不全或药理学抑制。这种“反弹”响应是通过独特的机制实现的。Nrf1组成型易位到ER内腔,N-糖基化,然后通过ER相关降解(ERAD)途径靶向进行蛋白酶体降解。蛋白酶体抑制导致胞浆Nrf1积累,然后将其加工形成活性转录因子。在这里,我们显示了胞质酶N-聚糖酶1(NGLY1,人PNGase)对于Nrf1激活对蛋白酶体抑制起着至关重要的作用。NGLY1活性的化学或遗传破坏会导致错误处理的Nrf1的积累,而Nrf1则在很大程度上被排除在细胞核之外。在这些条件下,Nrf1在调节蛋白酶体亚基时不响应蛋白酶体的抑制作用。通过小分子筛选,我们确定了一种破坏Nrf1加工和功能的具有细胞活性的NGLY1抑制剂。该化合物可增强卡非佐米(一种临床上使用的蛋白酶体抑制剂)对MM和T细胞衍生的急性淋巴细胞白血病(T-ALL)细胞系的细胞毒性。因此,


























 京公网安备 11010802027423号
京公网安备 11010802027423号