Journal of Catalysis ( IF 7.3 ) Pub Date : 2017-11-21 , DOI: 10.1016/j.jcat.2017.10.014 Sukaran S. Arora , Aditya Bhan
|
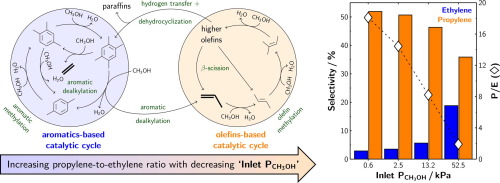
A monotonic increase (2–18) in the effluent propylene-to-ethylene molar ratio as inlet methanol pressure is varied from 52.5 to 0.6 kPa during methanol-to-hydrocarbons catalysis (∼30%C conversion) on H-ZSM-5 at 673 K reveals methanol pressure as the salient process parameter that allows control over the relative rates of propagation of the olefins- and aromatics-based methylation/cracking events. The enhanced propagation of the olefins-based cycle over its aromatics-based counterpart and consequently, decoupling of the two catalytic cycles at low influent methanol pressures is observed to persist irrespective of the reaction temperature (623–773 K). Reactions involving formaldehyde co-feeds (3–20 Pa or 0.5–5%C) with low-pressure (0.6 kPa) methanol at 623 K result in a monotonically decreasing trend in propylene-to-ethylene molar ratio from 24.7 in the absence of formaldehyde to 0.8 in the presence of 20 Pa formaldehyde implicating suppressed formaldehyde production from methanol transfer dehydrogenation events at low methanol pressures as the mechanistic basis for the observed effect of enhanced olefin cycle propagation. Co-reacting formaldehyde (11 Pa or 3%C) with propylene (0.1 kPa) on H-ZSM-5 at 623 K results in a 5.5-fold increase in aromatics selectivity suggesting Prins condensation reactions between formaldehyde and olefins are likely involved in aromatics production during methanol-to-hydrocarbons catalysis over H-ZSM-5.
中文翻译:

甲醇压力在控制其转移脱氢中的关键作用以及在H-ZSM-5上甲醇/烃催化过程中对丙烯/乙烯比的相应影响
在H-ZSM-5上,在甲醇转化为碳氢化合物的催化过程中(约30%C转化),随着进料甲醇压力的变化,流出的丙烯与乙烯的摩尔比单调增加(2-18)从52.5变为0.6 kPa。 673 K揭示了甲醇压力作为重要的工艺参数,可以控制基于烯烃和芳族化合物的甲基化/裂解事件的相对扩散速率。观察到基于烯烃的循环在其基于芳烃的对应物上的传播增加,因此,无论反应温度如何(623-773 K),都可以观察到在低进水甲醇压力下两个催化循环的解偶联仍然持续。涉及甲醛共进料(3–20 Pa或0.5–5%C)与低压(0.6 kPa)甲醇在623 K下的反应会导致丙烯与乙烯的摩尔比从24单调下降。在不存在甲醛的情况下,将图7中的甲醛在20Pa的存在下至0.8的影响暗示了在低甲醇压力下由甲醇转移脱氢事件引起的甲醛产生的抑制,作为观察到的增强的烯烃循环传播作用的机理基础。H-ZSM-5上在623 K下与丙烯(0.1 kPa)与丙烯(11 Pa或3%C)共反应会导致芳烃选择性增加5.5倍,这表明甲醛与烯烃之间的Prins缩合反应可能与芳烃有关H-ZSM-5催化甲醇制碳氢化合物过程中的合成 8在20 Pa甲醛存在下,暗示在低甲醇压力下由甲醇转移脱氢事件引起的甲醛生产受到抑制,这是观察到的增强烯烃循环扩散作用的机理基础。H-ZSM-5上在623 K下与丙烯(0.1 kPa)与丙烯(11 Pa或3%C)共反应会导致芳烃选择性增加5.5倍,这表明甲醛与烯烃之间的Prins缩合反应可能与芳烃有关H-ZSM-5催化甲醇制碳氢化合物过程中的合成 8在20 Pa甲醛存在下,暗示在低甲醇压力下由甲醇转移脱氢事件引起的甲醛生产受到抑制,这是观察到的增强烯烃循环扩散作用的机理基础。H-ZSM-5上在623 K时将甲醛(11 Pa或3%C)与丙烯(11 kPa或3%C)共反应会导致芳烃选择性增加5.5倍,这表明甲醛与烯烃之间的Prins缩合反应可能与芳烃有关H-ZSM-5催化甲醇制碳氢化合物过程中的合成



























 京公网安备 11010802027423号
京公网安备 11010802027423号