当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Early Development Scale-Up of a Novel CXCR Antagonist: Focus on Racemic and Stereoselective Routes of a Key Intermediate
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-11-29 00:00:00 , DOI: 10.1021/acs.oprd.7b00325 Samuel Tabet 1 , Nicolas Rodeville 1 , Arnaud Mathieu 1 , Catherine Raffin 1 , Corinne Millois-Barbuis 1 , Branislav Musicki 1 , Franck Muller 1 , Thibaud Gerfaud 1 , Jean-Guy Boiteau 1 , Isabelle Cardinaud 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2017-11-29 00:00:00 , DOI: 10.1021/acs.oprd.7b00325 Samuel Tabet 1 , Nicolas Rodeville 1 , Arnaud Mathieu 1 , Catherine Raffin 1 , Corinne Millois-Barbuis 1 , Branislav Musicki 1 , Franck Muller 1 , Thibaud Gerfaud 1 , Jean-Guy Boiteau 1 , Isabelle Cardinaud 1
Affiliation
|
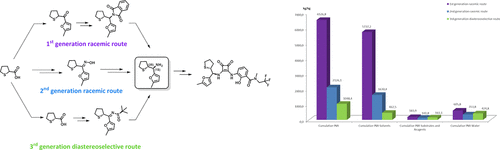
Efforts toward a convenient and scalable process for the synthesis of a novel CXCR antagonist 1 are described, with a specific focus on a chiral key intermediate. Two generations of a racemic route have been developed for short-term deliveries, and a stereoselective process has been devised for longer term plans. Key steps involved an enzymatic resolution of racemic tetrahydrothiophene-2-carboxylic acid to install the (2R) stereocenter, the mild and efficient preparation of a sterically hindered sulfinimine under a nitrogen flow, and its stereoselective reduction to set up the (1S) amine stereocenter. The process has been scaled-up to multikilogram scale for the racemic approach, and the stereoselective route was demonstrated on multigram scale.
中文翻译:

新型CXCR拮抗剂的早期开发规模扩大:重点研究关键中间体的外消旋和立体选择性途径
描述了对合成新型CXCR拮抗剂1的方便且可扩展的方法的努力,特别着重于手性关键中间体。已经开发出两代外消旋路线用于短期交付,并且已经为长期计划设计了立体选择过程。关键步骤涉及外消旋四氢噻吩-2-羧酸的酶促拆分,以建立(2 R)立体中心,在氮气流下温和有效地制备位阻亚氨嘧啶,及其立体选择性还原以建立(1 S)胺立体中心。对于外消旋方法,该过程已扩大到几千克规模,而立体选择性路线已在多克规模上得到证明。
更新日期:2017-11-29
中文翻译:

新型CXCR拮抗剂的早期开发规模扩大:重点研究关键中间体的外消旋和立体选择性途径
描述了对合成新型CXCR拮抗剂1的方便且可扩展的方法的努力,特别着重于手性关键中间体。已经开发出两代外消旋路线用于短期交付,并且已经为长期计划设计了立体选择过程。关键步骤涉及外消旋四氢噻吩-2-羧酸的酶促拆分,以建立(2 R)立体中心,在氮气流下温和有效地制备位阻亚氨嘧啶,及其立体选择性还原以建立(1 S)胺立体中心。对于外消旋方法,该过程已扩大到几千克规模,而立体选择性路线已在多克规模上得到证明。



























 京公网安备 11010802027423号
京公网安备 11010802027423号