Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ru-NHC Catalyzed Domino Reaction of Carbonyl Compounds and Alcohols: A Short Synthesis of Donaxaridine
ACS Omega ( IF 4.1 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsomega.7b01152 Girish Singh Bisht 1 , Moreshwar Bhagwan Chaudhari 1 , Vruta Sunil Gupte 1 , Boopathy Gnanaprakasam 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acsomega.7b01152 Girish Singh Bisht 1 , Moreshwar Bhagwan Chaudhari 1 , Vruta Sunil Gupte 1 , Boopathy Gnanaprakasam 1
Affiliation
|
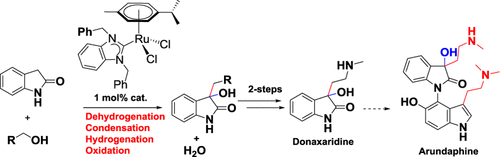
Direct α-alkylation of amides and the synthesis of C3-alkylated 3-hydroxyindolin-2-one/2-substituted-2-hydroxy-3,4-dihydronaphthalen-1(2H)-one derivatives from 2-oxindole/1-teralone were reported using primary alcohols in the presence of Ru-NHC catalyst in a one pot condition under the borrowing hydrogen method. In the case of inert conditions, 2-oxindole/1-teralone exclusively forms the C3-alkylated product. Whereas, allowing this reaction mixture to occur under an air atmosphere predominantly forms C3-alkylated 3-hydroxyindolin-2-one via domino C–H alkylation and aerobic C–H hydroxylation. This Ru-NHC catalyst is easily accessible, air stable, and phosphine free. Using this method, synthesis of 2-oxindole based natural products such as Donaxaridine was accomplished.
中文翻译:

Ru-NHC催化的羰基化合物与醇的多米诺反应:Donaxaridine的短合成
酰胺的直接α-烷基化反应和由2-oxindole / 1-合成C3-烷基化的3-hydroxyindolin-2-one / 2-取代的-2-hydroxy-3,4-dihydronaphthalen-1(2 H)-one衍生物据报道,在Ru-NHC催化剂存在下,在借用氢气法的一锅条件下,使用伯醇使用了三氢萘酮。在惰性条件下,2-oxindole / 1-teralone专门形成C3-烷基化产物。而使这种反应混合物在空气气氛下发生,主要是通过多米诺骨牌C–H烷基化和有氧C–H羟基化形成C3-烷基化的3-羟基吲哚-2-酮。这种Ru-NHC催化剂易于获取,空气稳定且不含膦。使用该方法,完成了基于2-氧吲哚的天然产物例如多那西啶的合成。
更新日期:2017-11-20
中文翻译:

Ru-NHC催化的羰基化合物与醇的多米诺反应:Donaxaridine的短合成
酰胺的直接α-烷基化反应和由2-oxindole / 1-合成C3-烷基化的3-hydroxyindolin-2-one / 2-取代的-2-hydroxy-3,4-dihydronaphthalen-1(2 H)-one衍生物据报道,在Ru-NHC催化剂存在下,在借用氢气法的一锅条件下,使用伯醇使用了三氢萘酮。在惰性条件下,2-oxindole / 1-teralone专门形成C3-烷基化产物。而使这种反应混合物在空气气氛下发生,主要是通过多米诺骨牌C–H烷基化和有氧C–H羟基化形成C3-烷基化的3-羟基吲哚-2-酮。这种Ru-NHC催化剂易于获取,空气稳定且不含膦。使用该方法,完成了基于2-氧吲哚的天然产物例如多那西啶的合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号