当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of a Novel and Selective Indoleamine 2,3-Dioxygenase (IDO-1) Inhibitor 3-(5-Fluoro-1H-indol-3-yl)pyrrolidine-2,5-dione (EOS200271/PF-06840003) and Its Characterization as a Potential Clinical Candidate
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00974 Stefano Crosignani 1 , Patrick Bingham 2 , Pauline Bottemanne 1 , Hélène Cannelle 1 , Sandra Cauwenberghs 1 , Marie Cordonnier 1 , Deepak Dalvie 2 , Frederik Deroose 3 , Jun Li Feng 2 , Bruno Gomes 1 , Samantha Greasley 2 , Stephen E Kaiser 2 , Manfred Kraus 2 , Michel Négrerie 4 , Karen Maegley 2 , Nichol Miller 2 , Brion W Murray 2 , Manfred Schneider 1 , James Soloweij 2 , Albert E Stewart 2 , Joseph Tumang 2 , Vince R Torti 2 , Benoit Van Den Eynde 5 , Martin Wythes 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jmedchem.7b00974 Stefano Crosignani 1 , Patrick Bingham 2 , Pauline Bottemanne 1 , Hélène Cannelle 1 , Sandra Cauwenberghs 1 , Marie Cordonnier 1 , Deepak Dalvie 2 , Frederik Deroose 3 , Jun Li Feng 2 , Bruno Gomes 1 , Samantha Greasley 2 , Stephen E Kaiser 2 , Manfred Kraus 2 , Michel Négrerie 4 , Karen Maegley 2 , Nichol Miller 2 , Brion W Murray 2 , Manfred Schneider 1 , James Soloweij 2 , Albert E Stewart 2 , Joseph Tumang 2 , Vince R Torti 2 , Benoit Van Den Eynde 5 , Martin Wythes 2
Affiliation
|
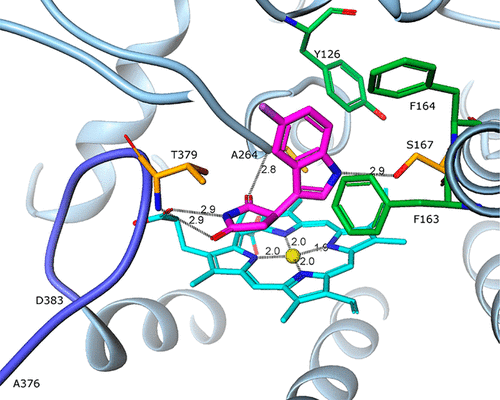
Tumors use tryptophan-catabolizing enzymes such as indoleamine 2,3-dioxygenase (IDO-1) to induce an immunosuppressive environment. IDO-1 is induced in response to inflammatory stimuli and promotes immune tolerance through effector T-cell anergy and enhanced Treg function. As such, IDO-1 is a nexus for the induction of a key immunosuppressive mechanism and represents an important immunotherapeutic target in oncology. Starting from HTS hit 5, IDO-1 inhibitor 6 (EOS200271/PF-06840003) has been developed. The structure–activity relationship around 6 is described and rationalized using the X-ray crystal structure of 6 bound to human IDO-1, which shows that 6, differently from most of the IDO-1 inhibitors described so far, does not bind to the heme iron atom and has a novel binding mode. Clinical candidate 6 shows good potency in an IDO-1 human whole blood assay and also shows a very favorable ADME profile leading to favorable predicted human pharmacokinetic properties, including a predicted half-life of 16–19 h.
中文翻译:

新型和选择性吲哚胺2,3-二加氧酶(IDO-1)抑制剂3-(5-氟-1 H-吲哚-3-基)吡咯烷-2,5-二酮(EOS200271 / PF-06840003)的发现及其表征为潜在的临床候选者
肿瘤使用色氨酸分解酶,例如吲哚胺2,3-二加氧酶(IDO-1)来诱导免疫抑制环境。IDO-1响应炎症刺激而被诱导,并通过效应T细胞无反应性和增强的Treg功能促进免疫耐受。因此,IDO-1是诱导关键免疫抑制机制的纽带,并代表肿瘤学中重要的免疫治疗靶标。从HTS 5开始,已开发出IDO-1抑制剂6(EOS200271 / PF-06840003)。周围的结构-活性关系6中描述和合理化使用的X射线晶体结构6结合人IDO-1,这表明6与迄今为止描述的大多数IDO-1抑制剂不同,它不与血红素铁原子结合,并具有新颖的结合方式。临床候选药物6在IDO-1人全血检测中显示出良好的功效,并且还显示出非常有利的ADME谱,从而导致有利的预期人药代动力学特性,包括16-19 h的预期半衰期。
更新日期:2017-11-21
中文翻译:

新型和选择性吲哚胺2,3-二加氧酶(IDO-1)抑制剂3-(5-氟-1 H-吲哚-3-基)吡咯烷-2,5-二酮(EOS200271 / PF-06840003)的发现及其表征为潜在的临床候选者
肿瘤使用色氨酸分解酶,例如吲哚胺2,3-二加氧酶(IDO-1)来诱导免疫抑制环境。IDO-1响应炎症刺激而被诱导,并通过效应T细胞无反应性和增强的Treg功能促进免疫耐受。因此,IDO-1是诱导关键免疫抑制机制的纽带,并代表肿瘤学中重要的免疫治疗靶标。从HTS 5开始,已开发出IDO-1抑制剂6(EOS200271 / PF-06840003)。周围的结构-活性关系6中描述和合理化使用的X射线晶体结构6结合人IDO-1,这表明6与迄今为止描述的大多数IDO-1抑制剂不同,它不与血红素铁原子结合,并具有新颖的结合方式。临床候选药物6在IDO-1人全血检测中显示出良好的功效,并且还显示出非常有利的ADME谱,从而导致有利的预期人药代动力学特性,包括16-19 h的预期半衰期。


























 京公网安备 11010802027423号
京公网安备 11010802027423号