当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Driving Force for the Association of Gemini Surfactants
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08936 Kyeong-Jun Jeong 1 , Arun Yethiraj 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08936 Kyeong-Jun Jeong 1 , Arun Yethiraj 1
Affiliation
|
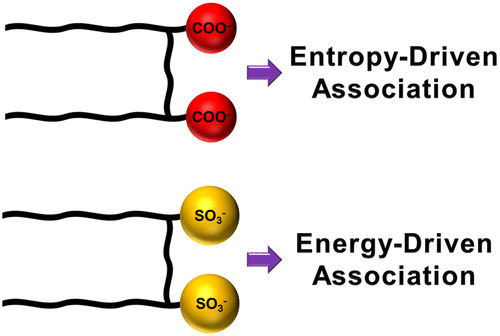
The self-assembly of surfactants into lyotropic liquid crystalline phases is interesting from a fundamental and practical perspective. The propensity for self-assembly is particularly interesting in Gemini surfactants which have a very low critical micelle concentration. In this work, we study the effect of headgroup identity on the driving force for the self-assembly of Gemini surfactants, using computer simulations of the potential of mean force (PMF). We find that surfactants with sulfonate headgroups have a greater tendency to assemble than those with carboxylate headgroups. The minimum in the PMF is about a factor of 2 deeper and occurs at shorter distances. Interestingly, the driving force is entropic with the carobxylate and energetic with the sulfonate headgroups. Analysis of different contributions suggests that these differences arise from surfactant headgroup electrostatics and size. The results provide an explanation for why the morphology diagram of the sulfonate surfactants is insensitive to temperature.
中文翻译:

双子表面活性剂协会的推动力
从基本和实用的角度看,表面活性剂自组装成溶致液晶相是令人感兴趣的。在具有非常低的临界胶束浓度的双子表面活性剂中,自组装的倾向特别令人感兴趣。在这项工作中,我们使用平均力潜势(PMF)的计算机模拟研究了头基同一性对Gemini表面活性剂自组装驱动力的影响。我们发现具有磺酸根头基的表面活性剂比具有羧酸根头基的表面活性剂具有更大的组装趋势。PMF中的最小值大约深2倍,并且发生在更短的距离处。有趣的是,驱动力与羰基化物是熵,而与磺酸盐基团是高能的。对不同贡献的分析表明,这些差异源于表面活性剂头基的静电和大小。结果解释了为什么磺酸盐表面活性剂的形态图对温度不敏感。
更新日期:2017-11-21
中文翻译:

双子表面活性剂协会的推动力
从基本和实用的角度看,表面活性剂自组装成溶致液晶相是令人感兴趣的。在具有非常低的临界胶束浓度的双子表面活性剂中,自组装的倾向特别令人感兴趣。在这项工作中,我们使用平均力潜势(PMF)的计算机模拟研究了头基同一性对Gemini表面活性剂自组装驱动力的影响。我们发现具有磺酸根头基的表面活性剂比具有羧酸根头基的表面活性剂具有更大的组装趋势。PMF中的最小值大约深2倍,并且发生在更短的距离处。有趣的是,驱动力与羰基化物是熵,而与磺酸盐基团是高能的。对不同贡献的分析表明,这些差异源于表面活性剂头基的静电和大小。结果解释了为什么磺酸盐表面活性剂的形态图对温度不敏感。



























 京公网安备 11010802027423号
京公网安备 11010802027423号