当前位置:
X-MOL 学术
›
J. Chem. Educ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Geometrical Description of Chemical Equilibrium and Le Châtelier’s Principle: Two-Component Systems
Journal of Chemical Education ( IF 3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jchemed.7b00665 Igor Novak 1
Journal of Chemical Education ( IF 3 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.jchemed.7b00665 Igor Novak 1
Affiliation
|
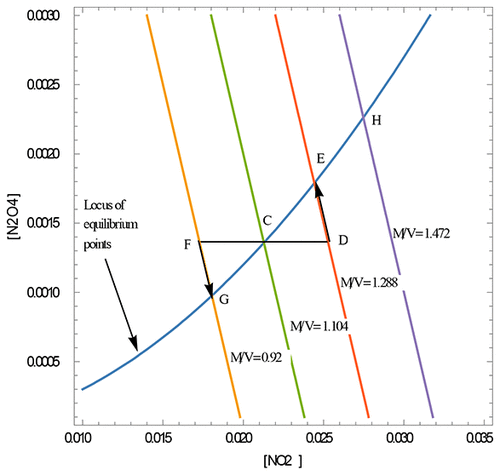
Chemical equilibrium is one of the most important concepts in chemistry. The changes in properties of the chemical system at equilibrium induced by variations in pressure, volume, temperature, and concentration are always included in classroom teaching and discussions. This work introduces a novel, geometrical approach to understanding the chemical system at equilibrium with the example of a simple two-component system and shows how the equilibrium changes under the influence of external perturbations. The paper discusses how thermodynamic factors (contained in the equilibrium constant K) and the conservation of mass principle govern the equilibrium state. The equilibrium and its changes are described using the geometrical representation in concentration space. The goal of this work is to help students better understand the basis behind the well-known Le Châtelier’s principle and equilibrium in general.
中文翻译:

化学平衡的几何描述和勒沙泰勒原理:两组分体系
化学平衡是化学中最重要的概念之一。由压力,体积,温度和浓度的变化引起的化学系统在平衡状态下的性能变化始终包含在课堂教学和讨论中。这项工作引入了一种新颖的几何方法,以简单的两组分系统为例来了解处于平衡状态的化学系统,并展示了平衡如何在外部扰动的影响下发生变化。本文讨论了热力学因素如何(包含在平衡常数K中)和质量守恒原理控制着平衡状态。使用浓度空间中的几何表示来描述平衡及其变化。这项工作的目的是帮助学生更好地理解众所周知的勒沙特里耶原理和均衡的基本原理。
更新日期:2017-11-17
中文翻译:

化学平衡的几何描述和勒沙泰勒原理:两组分体系
化学平衡是化学中最重要的概念之一。由压力,体积,温度和浓度的变化引起的化学系统在平衡状态下的性能变化始终包含在课堂教学和讨论中。这项工作引入了一种新颖的几何方法,以简单的两组分系统为例来了解处于平衡状态的化学系统,并展示了平衡如何在外部扰动的影响下发生变化。本文讨论了热力学因素如何(包含在平衡常数K中)和质量守恒原理控制着平衡状态。使用浓度空间中的几何表示来描述平衡及其变化。这项工作的目的是帮助学生更好地理解众所周知的勒沙特里耶原理和均衡的基本原理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号