当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Single Quantum Dot-Based Nanosensor for Sensitive Detection of O-GlcNAc Transferase Activity
Analytical Chemistry ( IF 7.4 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.analchem.7b04065 Juan Hu 1 , Yueying Li 1 , Ying Li 2 , Bo Tang 1 , Chun-yang Zhang 1
Analytical Chemistry ( IF 7.4 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acs.analchem.7b04065 Juan Hu 1 , Yueying Li 1 , Ying Li 2 , Bo Tang 1 , Chun-yang Zhang 1
Affiliation
|
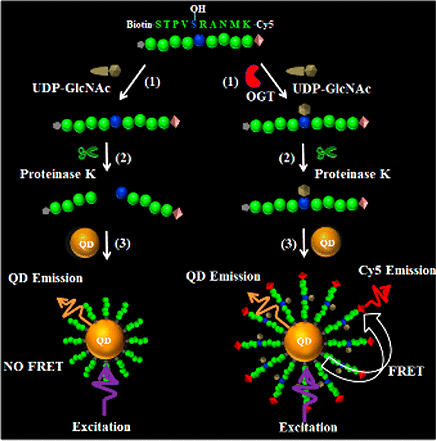
Protein glycosylation is a ubiquitous post-translational modification that plays crucial roles in modulating biological recognition events in development and physiology. Human O-GlcNAc transferase (OGT) is an intracellular enzyme responsible for O-linked N-acetylglucosamine (O-GlcNAc) glycosylation, and the deregulation of OGT activity occurs in cancer, diabetes, and neurodegenerative disease. Here we develop a single quantum dot (QD)-based nanosensor for sensitive OGT assay. We design a Cy5/biotin-modified peptide with a serine hydroxyl group for sensing OGT and a protease site adjacent to the glycosylation site for proteinase cleavage, with a universal nonradioactive UDP-GlcNAc as the sugar donor and a Cy5/biotin-modified peptide as the substrate. In the presence of OGT, it catalyzes the glycosylation reaction to generate a glycosylated peptide that is a protease-protection peptide. The resultant glycosylated Cy5/biotin-modified peptides may assemble on the surface of the streptavidin-coated QD to obtain a QD–peptide–Cy5 nanostructure in which the fluorescence resonance energy transfer (FRET) from the QD to Cy5 can occur, leading to the emission of Cy5 which can be quantified by single-molecule detection. This method exhibits high sensitivity with a limit of detection of 3.47 × 10–13 M, and it is very simple and straightforward without the involvement of any enzyme purification, radioisotope-labeled sugar donors, specific antibodies, and the synthesis of fluorescent UDP-GlcNAc analogues. Moreover, this method can be used for enzyme kinetic analysis, quantitative detection of cellular OGT activity, and the screening of OGT inhibitors, holding great potential for further application in drug discovery and clinical diagnosis.
中文翻译:

基于单个量子点的纳米传感器,用于O-GlcNAc转移酶活性的灵敏检测
蛋白质糖基化是一种普遍存在的翻译后修饰,在调节发育和生理学中的生物识别事件中起着至关重要的作用。人O-GlcNAc转移酶(OGT)是负责O连锁N的细胞内酶-乙酰氨基葡萄糖(O-GlcNAc)糖基化,OGT活性的失调发生在癌症,糖尿病和神经退行性疾病中。在这里,我们开发了一种基于单量子点(QD)的纳米传感器,用于敏感的OGT分析。我们设计了一个具有丝氨酸羟基的Cy5 /生物素修饰的肽,用于检测OGT和一个邻近糖基化位点的蛋白酶位点,用于蛋白酶的切割,其中一种通用的非放射性UDP-GlcNAc作为糖供体,而一个Cy5 /生物素修饰的肽为基板。在OGT的存在下,它催化糖基化反应以生成糖基化肽,它是一种蛋白酶保护肽。由此产生的糖基化Cy5 /生物素修饰肽可在抗生蛋白链菌素包被的QD的表面组装,以获得QD-肽-Cy5纳米结构,其中可发生从QD到Cy5的荧光共振能量转移(FRET),从而导致可以通过单分子检测定量的Cy5的发射。该方法灵敏度高,检出限为3.47×10–13 M,它非常简单明了,无需任何酶纯化,放射性同位素标记的糖供体,特异性抗体和荧光UDP-GlcNAc类似物的合成。而且,该方法可用于酶动力学分析,细胞OGT活性的定量检测以及OGT抑制剂的筛选,具有在药物开发和临床诊断中进一步应用的巨大潜力。
更新日期:2017-11-19
中文翻译:

基于单个量子点的纳米传感器,用于O-GlcNAc转移酶活性的灵敏检测
蛋白质糖基化是一种普遍存在的翻译后修饰,在调节发育和生理学中的生物识别事件中起着至关重要的作用。人O-GlcNAc转移酶(OGT)是负责O连锁N的细胞内酶-乙酰氨基葡萄糖(O-GlcNAc)糖基化,OGT活性的失调发生在癌症,糖尿病和神经退行性疾病中。在这里,我们开发了一种基于单量子点(QD)的纳米传感器,用于敏感的OGT分析。我们设计了一个具有丝氨酸羟基的Cy5 /生物素修饰的肽,用于检测OGT和一个邻近糖基化位点的蛋白酶位点,用于蛋白酶的切割,其中一种通用的非放射性UDP-GlcNAc作为糖供体,而一个Cy5 /生物素修饰的肽为基板。在OGT的存在下,它催化糖基化反应以生成糖基化肽,它是一种蛋白酶保护肽。由此产生的糖基化Cy5 /生物素修饰肽可在抗生蛋白链菌素包被的QD的表面组装,以获得QD-肽-Cy5纳米结构,其中可发生从QD到Cy5的荧光共振能量转移(FRET),从而导致可以通过单分子检测定量的Cy5的发射。该方法灵敏度高,检出限为3.47×10–13 M,它非常简单明了,无需任何酶纯化,放射性同位素标记的糖供体,特异性抗体和荧光UDP-GlcNAc类似物的合成。而且,该方法可用于酶动力学分析,细胞OGT活性的定量检测以及OGT抑制剂的筛选,具有在药物开发和临床诊断中进一步应用的巨大潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号