当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Importance of Ag–Cu Biphasic Boundaries for Selective Electrochemical Reduction of CO2 to Ethanol
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acscatal.7b02822 Seunghwa Lee 1 , Gibeom Park 1 , Jaeyoung Lee 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acscatal.7b02822 Seunghwa Lee 1 , Gibeom Park 1 , Jaeyoung Lee 1
Affiliation
|
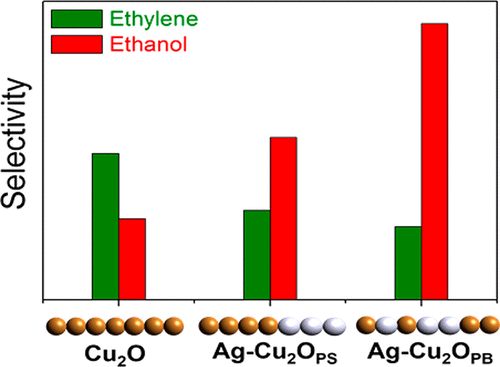
In recent years, electrochemical reduction of carbon dioxide (CO2) has received a great deal of attention due to the potential that this process can mitigate the atmospheric CO2 concentration and produce valuable organic compounds. In particular, Cu and Cu-based catalysts have exhibited the capability of converting CO2 into multicarbon fuels and chemicals in significant quantities. Here, we report a facile and cheap fabrication method for the development of an Ag-incorporated cuprous oxide (Ag-Cu2O) electrode enabling selective synthesis of ethanol via electrochemical CO2 reduction and reveal the key factor improving the ethanol (C2H5OH) selectivity. The incorporation of Ag into Cu2O leads to the suppression of hydrogen (H2) evolution, and furthermore, by varying the elemental arrangement (phase-separated and phase-blended) of Ag and Cu, we observe that C2H5OH selectivity can be controlled. Consequently, the Faradaic efficiency for C2H5OH on phase-blended Ag-Cu2O (Ag-Cu2OPB) is 3 times higher than that of the Cu2O without Ag dopant. We propose that the electrochemical reaction behavior is not solely associated with a role of Ag dopant, carbon monoxide (CO) leading to an ethanol formation pathway over ethylene, but also the doping pattern related population of Ag-Cu biphasic boundaries relatively suppresses the H2 evolution reaction and encourages the reaction of mobile CO generated on Ag to a residual intermediate on a Cu site.
中文翻译:

Ag-Cu两相边界对于选择性电化学还原CO 2转化为乙醇的重要性
近年来,由于电化学还原二氧化碳(CO 2)可以减轻大气中CO 2的浓度并产生有价值的有机化合物,因此备受关注。特别地,Cu和Cu基催化剂已经表现出将CO 2大量转化为多碳燃料和化学品的能力。在这里,我们报告了一种易于开发且便宜的制造方法,用于开发结合有银的氧化亚铜(Ag-Cu 2 O)电极,该电极能够通过电化学CO 2还原选择性地合成乙醇,并揭示了改善乙醇(C 2 H 5OH)的选择性。将Ag掺入Cu 2 O可以抑制氢(H 2)的释放,此外,通过改变Ag和Cu的元素排列(相分离和相混合),我们观察到C 2 H 5 OH选择性可以控制。因此,法拉第效率对C 2 H ^ 5 OH上相共混的Ag-Cu系2 O(Ag-Cu系2 ö PB)比铜的高3倍2O,不含Ag掺杂剂。我们建议,电化学反应行为不仅与银掺杂剂,一氧化碳(CO)导致乙烯上的乙醇形成途径的作用有关,而且与掺杂模式相关的银铜双相边界总体可相对抑制H 2。生成反应,并鼓励在银上生成的可移动CO与铜位点上的残留中间体发生反应。
更新日期:2017-11-19
中文翻译:

Ag-Cu两相边界对于选择性电化学还原CO 2转化为乙醇的重要性
近年来,由于电化学还原二氧化碳(CO 2)可以减轻大气中CO 2的浓度并产生有价值的有机化合物,因此备受关注。特别地,Cu和Cu基催化剂已经表现出将CO 2大量转化为多碳燃料和化学品的能力。在这里,我们报告了一种易于开发且便宜的制造方法,用于开发结合有银的氧化亚铜(Ag-Cu 2 O)电极,该电极能够通过电化学CO 2还原选择性地合成乙醇,并揭示了改善乙醇(C 2 H 5OH)的选择性。将Ag掺入Cu 2 O可以抑制氢(H 2)的释放,此外,通过改变Ag和Cu的元素排列(相分离和相混合),我们观察到C 2 H 5 OH选择性可以控制。因此,法拉第效率对C 2 H ^ 5 OH上相共混的Ag-Cu系2 O(Ag-Cu系2 ö PB)比铜的高3倍2O,不含Ag掺杂剂。我们建议,电化学反应行为不仅与银掺杂剂,一氧化碳(CO)导致乙烯上的乙醇形成途径的作用有关,而且与掺杂模式相关的银铜双相边界总体可相对抑制H 2。生成反应,并鼓励在银上生成的可移动CO与铜位点上的残留中间体发生反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号