当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
DNA Aptamer-Based Activatable Probes for Photoacoustic Imaging in Living Mice
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/jacs.7b07913 Jingjing Zhang 1 , Lukas P. Smaga 1 , Nitya Sai Reddy Satyavolu 1 , Jefferson Chan 1 , Yi Lu 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/jacs.7b07913 Jingjing Zhang 1 , Lukas P. Smaga 1 , Nitya Sai Reddy Satyavolu 1 , Jefferson Chan 1 , Yi Lu 1
Affiliation
|
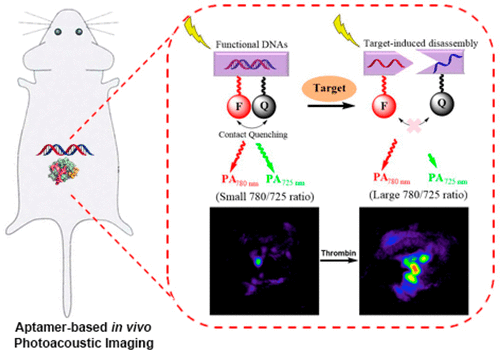
DNA aptamers are a powerful class of molecules for sensing targets, but have been limited when applied to imaging in living animals because most aptamer probes are fluorescence-based, which limits imaging penetration depth. Photoacoustic (PA) imaging emerged as an alternative to MRI and X-ray tomography in biomedical imaging, due to its ability to afford high-resolution images at depths in the cm range. Despite its promise, PA imaging is limited by a lack of strategies to design selective and activatable probes for targets. To overcome this limitation, we report design and demonstration of PA probes based on DNA aptamers that can hybridize to DNA strands conjugated to a near-infrared fluorophore/quencher pair (IRDye 800CW/IRDye QC-1) with efficient contact quenching. Binding of the target triggered a release of the DNA strand with the quencher and thus relief of the contact quenching, resulting in a change of the PA signal ratio at 780/725 nm. Using thrombin as a model, a relationship was established between the thrombin concentrations and the PA ratio, with a dynamic range of 0–1000 nM and a limit of detection of 112 nM. Finally, in vivo PA imaging studies showed that the PA ratio increased significantly 45 min after injection of thrombin but not with injection of PBS as a vehicle control, demonstrating the first aptamer-based activatable PA probe for advanced molecular imaging in living mice. Since in vitro selection can obtain aptamers selective for many targets, the design demonstrated can be applied for PA imaging of a number of targets.
中文翻译:

基于DNA适体的可激活探针用于活体小鼠的光声成像。
DNA适体是一类功能强大的分子,可感应靶标,但在应用于活体动物成像时受到限制,因为大多数适体探针都是基于荧光的,从而限制了成像的穿透深度。由于光声(PA)能够提供厘米级深度的高分辨率图像,因此已成为生物医学成像中MRI和X射线断层扫描的替代方法。尽管有前途,但PA成像技术由于缺乏针对靶标设计选择性和可激活探针的策略而受到限制。为了克服这一局限性,我们报告了基于DNA适体的PA探针的设计和演示,该适体可以与具有有效接触淬灭作用的近红外荧光团/猝灭剂对(IRDye 800CW / IRDye QC-1)缀合的DNA链杂交。靶标的结合触发了DNA链与淬灭剂的释放,从而缓解了接触淬灭,从而导致780/725 nm处的PA信号比率发生变化。以凝血酶为模型,建立凝血酶浓度与PA比之间的关系,动态范围为0–1000 nM,检出限为112 nM。最后,体内PA成像研究表明,注射凝血酶后45分钟,PA比率显着增加,但未注射PBS作为媒介物对照,这表明活体小鼠中第一个用于适体分子成像的基于适体的可活化PA探针。由于体外选择可以获得对许多靶标具有选择性的适体,因此所证明的设计可用于许多靶标的PA成像。
更新日期:2017-11-19
中文翻译:

基于DNA适体的可激活探针用于活体小鼠的光声成像。
DNA适体是一类功能强大的分子,可感应靶标,但在应用于活体动物成像时受到限制,因为大多数适体探针都是基于荧光的,从而限制了成像的穿透深度。由于光声(PA)能够提供厘米级深度的高分辨率图像,因此已成为生物医学成像中MRI和X射线断层扫描的替代方法。尽管有前途,但PA成像技术由于缺乏针对靶标设计选择性和可激活探针的策略而受到限制。为了克服这一局限性,我们报告了基于DNA适体的PA探针的设计和演示,该适体可以与具有有效接触淬灭作用的近红外荧光团/猝灭剂对(IRDye 800CW / IRDye QC-1)缀合的DNA链杂交。靶标的结合触发了DNA链与淬灭剂的释放,从而缓解了接触淬灭,从而导致780/725 nm处的PA信号比率发生变化。以凝血酶为模型,建立凝血酶浓度与PA比之间的关系,动态范围为0–1000 nM,检出限为112 nM。最后,体内PA成像研究表明,注射凝血酶后45分钟,PA比率显着增加,但未注射PBS作为媒介物对照,这表明活体小鼠中第一个用于适体分子成像的基于适体的可活化PA探针。由于体外选择可以获得对许多靶标具有选择性的适体,因此所证明的设计可用于许多靶标的PA成像。



























 京公网安备 11010802027423号
京公网安备 11010802027423号