Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent Polarity-Dependent Behavior of Aliphatic Thiols and Amines toward Intriguingly Fluorescent AuAgGSH Assembly
ACS Omega ( IF 4.1 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsomega.7b01560 Jayasmita Jana 1 , Teresa Aditya 1 , Yuichi Negishi 2 , Tarasankar Pal 1
ACS Omega ( IF 4.1 ) Pub Date : 2017-11-17 00:00:00 , DOI: 10.1021/acsomega.7b01560 Jayasmita Jana 1 , Teresa Aditya 1 , Yuichi Negishi 2 , Tarasankar Pal 1
Affiliation
|
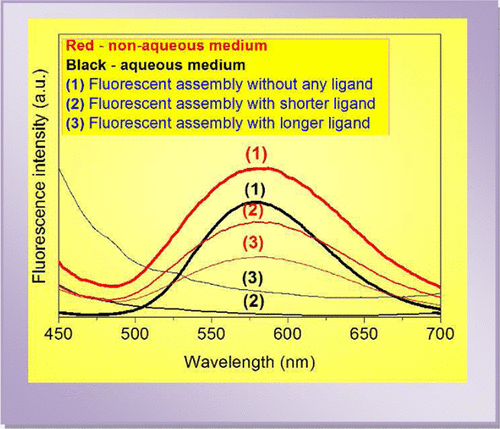
Highly stable fluorescent glutathione (GSH)-protected AuAg assembly has been synthesized in water under UV irradiation. The assembly is composed of small Ag2/Ag3 clusters. These clusters gain stability through synergistic interaction with Au(I) present within the assembly. This makes the overall assembly fluorescent. Here, GSH acts as a reducing as well as stabilizing agent. The assembly is so robust that it can be vacuum-dried to solid particles. The as-obtained solid is dispersible in nonaqueous solvents. The interaction between solvent and the assembly provides stability to the assembly, and the assembly shows fluorescence. It is interesting to see that the behavior of long-chain aliphatic thiols or amines toward the fluorescent assembly is altogether a different phenomenon in aqueous and nonaqueous mediums. The assembly gets ruptured in water due to direct interaction with long-chain thiols or amines, whereas in nonaqueous medium, solvation of added thiols or amines becomes pronounced, which hinders the interaction of solvent with the assembly. However, the fluorescence of the assembly is always quenched with thiols or amines no matter what the solvent medium is. In aqueous medium, the fluorescence quenching by aliphatic thiol or amine becomes pronounced with successive decrease in their chain length, whereas in nonaqueous medium, the trend is just reversed with chain length. The reasons behind such an interesting reversal of fluorescence quenching in aqueous and nonaqueous solvents have been discussed explicitly. Again, in organic solvents, thiol or amine-induced quenched fluorescence is selectively recovered by Pb(II) ion without any alteration of excitation and emission maxima. This phenomenon is not observed in water because of the ruptured fluorescent assembly. The fluorescence recovery by Pb(II) and unaltered emission peak only in nonaqueous solvent unequivocally prove the engagement of Pb(II) with thiols or amines, which in turn revert the original solvent-supported stabilization of the assembly.
中文翻译:

脂肪族硫醇和胺对迷人的荧光AuAgGSH组装体的溶剂极性依赖性行为
在紫外线照射下,已在水中合成了高度稳定的荧光谷胱甘肽(GSH)保护的AuAg组装体。组件由小型Ag 2 / Ag 3组成集群。这些簇通过与组件中存在的Au(I)协同相互作用获得稳定性。这使整个组件发荧光。在此,GSH既充当还原剂又充当稳定剂。该组件非常坚固,可以真空干燥成固体颗粒。所获得的固体可分散在非水溶剂中。溶剂与组件之间的相互作用为组件提供了稳定性,并且组件显示荧光。有趣的是,在水性和非水性介质中,长链脂族硫醇或胺对荧光组件的行为完全是不同的现象。由于与长链硫醇或胺的直接相互作用,装配体在水中破裂,而在非水介质中,添加的硫醇或胺的溶剂化变得明显,这阻碍了溶剂与组件的相互作用。但是,无论溶剂介质是什么,组件的荧光总是被硫醇或胺猝灭。在水性介质中,脂族硫醇或胺的荧光猝灭随着链长的连续减小而变得明显,而在非水性介质中,趋势随链长的变化正好相反。明确讨论了在水性和非水性溶剂中荧光猝灭如此有趣的逆转背后的原因。同样,在有机溶剂中,硫醇或胺诱导的猝灭荧光可通过Pb(II)离子选择性回收,而不会改变激发和发射最大值。由于荧光组件破裂,在水中未观察到此现象。
更新日期:2017-11-17
中文翻译:

脂肪族硫醇和胺对迷人的荧光AuAgGSH组装体的溶剂极性依赖性行为
在紫外线照射下,已在水中合成了高度稳定的荧光谷胱甘肽(GSH)保护的AuAg组装体。组件由小型Ag 2 / Ag 3组成集群。这些簇通过与组件中存在的Au(I)协同相互作用获得稳定性。这使整个组件发荧光。在此,GSH既充当还原剂又充当稳定剂。该组件非常坚固,可以真空干燥成固体颗粒。所获得的固体可分散在非水溶剂中。溶剂与组件之间的相互作用为组件提供了稳定性,并且组件显示荧光。有趣的是,在水性和非水性介质中,长链脂族硫醇或胺对荧光组件的行为完全是不同的现象。由于与长链硫醇或胺的直接相互作用,装配体在水中破裂,而在非水介质中,添加的硫醇或胺的溶剂化变得明显,这阻碍了溶剂与组件的相互作用。但是,无论溶剂介质是什么,组件的荧光总是被硫醇或胺猝灭。在水性介质中,脂族硫醇或胺的荧光猝灭随着链长的连续减小而变得明显,而在非水性介质中,趋势随链长的变化正好相反。明确讨论了在水性和非水性溶剂中荧光猝灭如此有趣的逆转背后的原因。同样,在有机溶剂中,硫醇或胺诱导的猝灭荧光可通过Pb(II)离子选择性回收,而不会改变激发和发射最大值。由于荧光组件破裂,在水中未观察到此现象。



























 京公网安备 11010802027423号
京公网安备 11010802027423号