当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
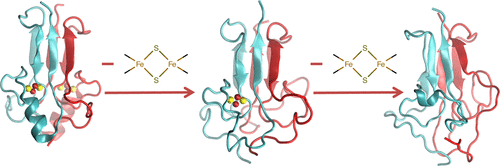
Molecular Dynamics Simulations of the [2Fe–2S] Cluster-Binding Domain of NEET Proteins Reveal Key Molecular Determinants That Induce Their Cluster Transfer/Release
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b10584 Luca Pesce 1 , Vania Calandrini 1 , Henri-baptiste Marjault 2 , Colin H. Lipper 3 , Gulia Rossetti 1, 4, 5 , Ron Mittler 6 , Patricia A. Jennings 3 , Andreas Bauer 7 , Rachel Nechushtai 2 , Paolo Carloni 1, 8
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.jpcb.7b10584 Luca Pesce 1 , Vania Calandrini 1 , Henri-baptiste Marjault 2 , Colin H. Lipper 3 , Gulia Rossetti 1, 4, 5 , Ron Mittler 6 , Patricia A. Jennings 3 , Andreas Bauer 7 , Rachel Nechushtai 2 , Paolo Carloni 1, 8
Affiliation
|
The NEET proteins are a novel family of iron–sulfur proteins characterized by an unusual three cysteine and one histidine coordinated [2Fe–2S] cluster. Aberrant cluster release, facilitated by the breakage of the Fe–N bond, is implicated in a variety of human diseases, including cancer. Here, the molecular dynamics in the multi-microsecond timescale, along with quantum chemical calculations, on two representative members of the family (the human NAF-1 and mitoNEET proteins), show that the loss of the cluster is associated with a dramatic decrease in secondary and tertiary structure. In addition, the calculations provide a mechanism for cluster release and clarify, for the first time, crucial differences existing between the two proteins, which are reflected in the experimentally observed difference in the pH-dependent cluster reactivity. The reliability of our conclusions is established by an extensive comparison with the NMR data of the solution proteins, in part measured in this work.
中文翻译:

NEET蛋白的[2Fe–2S]簇结合域的分子动力学模拟揭示了诱导其簇转移/释放的关键分子决定簇。
NEET蛋白是一个新的铁硫蛋白家族,其特征是具有不寻常的三个半胱氨酸和一个组氨酸配位的[2Fe-2S]簇。Fe-N键断裂促进异常簇释放,与包括癌症在内的多种人类疾病有关。在这里,在多微秒级的分子动力学以及量子化学计算,对该家族的两个代表性成员(人NAF-1和mitoNEET蛋白)进行了研究,结果表明,该簇的损失与H2O的急剧减少有关。二级和三级结构。另外,该计算提供了簇释放的机制,并首次澄清了两种蛋白质之间存在的关键差异,这在pH依赖性簇反应性的实验观察到的差异中得到反映。
更新日期:2017-11-17
中文翻译:

NEET蛋白的[2Fe–2S]簇结合域的分子动力学模拟揭示了诱导其簇转移/释放的关键分子决定簇。
NEET蛋白是一个新的铁硫蛋白家族,其特征是具有不寻常的三个半胱氨酸和一个组氨酸配位的[2Fe-2S]簇。Fe-N键断裂促进异常簇释放,与包括癌症在内的多种人类疾病有关。在这里,在多微秒级的分子动力学以及量子化学计算,对该家族的两个代表性成员(人NAF-1和mitoNEET蛋白)进行了研究,结果表明,该簇的损失与H2O的急剧减少有关。二级和三级结构。另外,该计算提供了簇释放的机制,并首次澄清了两种蛋白质之间存在的关键差异,这在pH依赖性簇反应性的实验观察到的差异中得到反映。



























 京公网安备 11010802027423号
京公网安备 11010802027423号