当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N,N′-Bisoxalamides Enhance the Catalytic Activity in Cu-Catalyzed Coupling of (Hetero)Aryl Bromides with Anilines and Secondary Amines
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.joc.7b02363 Subhajit Bhunia 1 , S. Vijay Kumar 1 , Dawei Ma 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-16 00:00:00 , DOI: 10.1021/acs.joc.7b02363 Subhajit Bhunia 1 , S. Vijay Kumar 1 , Dawei Ma 1
Affiliation
|
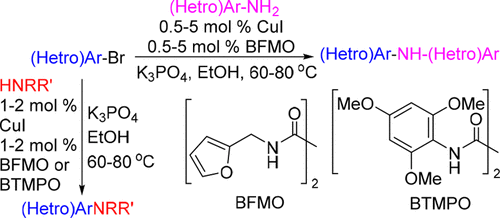
N,N′-Bis(furan-2-ylmethyl)oxalamide (BFMO), an inexpensive and conveniently available bidentate ligand, is very effective for promoting Cu-catalyzed N-arylation of anilines and cyclic secondary amines. The method enables coupling of a broad range of (hetero)aryl bromides with various (hetero)aryl amines and cyclic secondary amines at 0.5–5 mol % catalyst loadings at relatively low temperatures. For coupling with more sterically hindered acyclic secondary amines, using N,N′-bis(2,4,6-trimethoxyphenyl)oxalamide (BTMPO) as a ligand gives the better results. Additionally, high selectivity is achieved in CuI/BFMO-catalyzed direct monoarylation of piperazine with (hetero)aryl bromides to afford pharmaceutically important building blocks.
中文翻译:

N,N'-二异戊酰胺可增强(杂)芳基溴化物与苯胺和仲胺的铜催化偶联反应
N,N′-双(呋喃-2-基甲基)草酰胺(BFMO),一种廉价且方便获得的二齿配体,对于促进Cu催化的苯胺和环状仲胺的N-芳基化非常有效。该方法可以在相对较低的温度下以0.5-5 mol%的催化剂负载量将各种(杂)芳基溴化物与各种(杂)芳基胺和环状仲胺偶联。为了与更受空间阻碍的无环仲胺偶联,可使用N,N以'-双(2,4,6-三甲氧基苯基)草酰胺(BTMPO)为配体可获得更好的结果。另外,在CuI / BFMO催化的哌嗪与(杂)芳基溴化物直接单芳基化中获得了高选择性,从而获得了重要的药学组成部分。
更新日期:2017-11-16
中文翻译:

N,N'-二异戊酰胺可增强(杂)芳基溴化物与苯胺和仲胺的铜催化偶联反应
N,N′-双(呋喃-2-基甲基)草酰胺(BFMO),一种廉价且方便获得的二齿配体,对于促进Cu催化的苯胺和环状仲胺的N-芳基化非常有效。该方法可以在相对较低的温度下以0.5-5 mol%的催化剂负载量将各种(杂)芳基溴化物与各种(杂)芳基胺和环状仲胺偶联。为了与更受空间阻碍的无环仲胺偶联,可使用N,N以'-双(2,4,6-三甲氧基苯基)草酰胺(BTMPO)为配体可获得更好的结果。另外,在CuI / BFMO催化的哌嗪与(杂)芳基溴化物直接单芳基化中获得了高选择性,从而获得了重要的药学组成部分。



























 京公网安备 11010802027423号
京公网安备 11010802027423号