当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ruthenium-Catalyzed C–H Benzoxylation of tert-Benzamides with Aromatic Acids by Weak Coordination
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02495 Nagnath Yadav More 1 , Kishor Padala 1 , Masilamani Jeganmohan 1, 2
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.joc.7b02495 Nagnath Yadav More 1 , Kishor Padala 1 , Masilamani Jeganmohan 1, 2
Affiliation
|
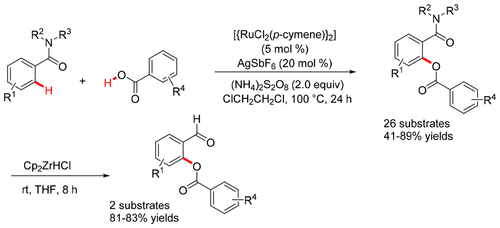
A highly regioselective C–H benzoxylation of tertiary benzamides with aromatic acids by weak O-amide coordination in the presence of [{RuCl2(p-cymene)}2], AgSbF6, and (NH4)2S2O8 in 1,2-dichloroethane at 100 °C for 24 h to afford ortho-benzoxylated tertiary benzamides is described. Selectively, ortho-benzoxylated cyclic benzamides were converted into ortho-benzoxylated benzaldehydes by using Cp2ZrHCl at room temperature. Subsequently, substituted salicylic acids were prepared by deprotection of the ester and amide groups of ortho-benzoxylated cyclic benzamides.
中文翻译:

弱配位的钌催化叔丁基酰胺与芳香酸的CH-H苄氧基化
在[{RuCl 2(p- Cymene)} 2 ],AgSbF 6和(NH 4)2 S 2 O 8存在的情况下,通过弱O-酰胺配位,高芳烃选择性地将叔苯甲酰胺与芳族酸进行C-H苯甲氧基化。描述了在100℃下1,2-二氯乙烷24小时以提供邻-苯甲酰化的叔苯甲酰胺。通过使用Cp 2选择性地将邻苯甲酰化的环状苯甲酰胺转化为邻苯甲酰化的苯甲醛ZrHCl在室温下。随后,通过使邻-苯甲酰化的环状苯甲酰胺的酯和酰胺基脱保护来制备取代的水杨酸。
更新日期:2017-11-16
中文翻译:

弱配位的钌催化叔丁基酰胺与芳香酸的CH-H苄氧基化
在[{RuCl 2(p- Cymene)} 2 ],AgSbF 6和(NH 4)2 S 2 O 8存在的情况下,通过弱O-酰胺配位,高芳烃选择性地将叔苯甲酰胺与芳族酸进行C-H苯甲氧基化。描述了在100℃下1,2-二氯乙烷24小时以提供邻-苯甲酰化的叔苯甲酰胺。通过使用Cp 2选择性地将邻苯甲酰化的环状苯甲酰胺转化为邻苯甲酰化的苯甲醛ZrHCl在室温下。随后,通过使邻-苯甲酰化的环状苯甲酰胺的酯和酰胺基脱保护来制备取代的水杨酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号