当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Interaction of SO2 with the Surface of a Water Nanodroplet
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b09900 Jie Zhong 1 , Chongqin Zhu 1 , Lei Li 1 , Geraldine L. Richmond 2 , Joseph S. Francisco 1 , Xiao Cheng Zeng 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2017-11-14 00:00:00 , DOI: 10.1021/jacs.7b09900 Jie Zhong 1 , Chongqin Zhu 1 , Lei Li 1 , Geraldine L. Richmond 2 , Joseph S. Francisco 1 , Xiao Cheng Zeng 1
Affiliation
|
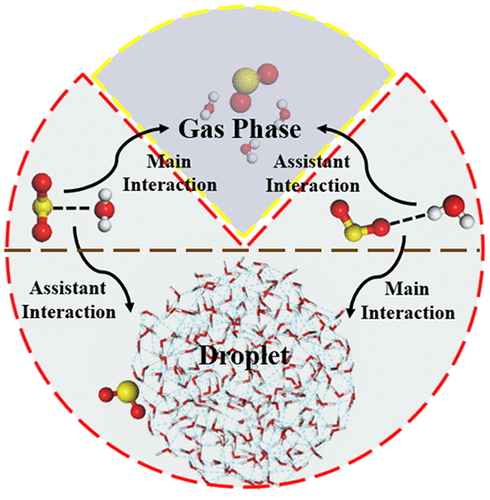
We present a comprehensive computational study of interaction of a SO2 with water molecules in the gas phase and with the surface of various sized water nanodroplets to investigate the solvation behavior of SO2 in different atmospheric environments. Born–Oppenheimer molecular dynamics (BOMD) simulation shows that, in the gas phase and at a temperature of 300 K, the dominant interaction between SO2 and H2O is (SO2)S···O(H2O), consistent with previous density-functional theory (DFT) computation at 0 K. However, at the surface of a water nanodroplet, BOMD simulation shows that the hydrogen-bonding interaction of (SO2)O···H(H2O) becomes increasingly important with the increase of droplet size, reflecting a marked effect of the water surface on the SO2 solvation. This conclusion is in good accordance with spectroscopy evidence obtained previously (J. Am. Chem. Soc. 2005, 127, 16806; J. Am. Chem. Soc. 2006, 128, 3256). The prevailing interaction (SO2)O···H(H2O) on a large droplet is mainly due to favorable exposure of H atoms of H2O at the air–water interface. Indeed, the conversion of the dominant interaction in the gas phase (SO2)S···O(H2O) to the dominant interaction on the water nanodroplet (SO2)O···H(H2O) may incur effects on the SO2 chemistry in atmospheric aerosols because the solvation of SO2 at the water surface can affect the reactive sites and electrophilicity of SO2. Hence, the solvation of SO2 on the aerosol surface may have new implications when studying SO2 chemistry in the aerosol-containing troposphere.
中文翻译:

SO 2与水纳米液滴表面的相互作用
我们提出了一种SO 2与气相中的水分子以及与各种尺寸的水纳米液滴表面相互作用的综合计算研究,以研究SO 2在不同大气环境中的溶剂化行为。Born-Oppenheimer分子动力学(BOMD)模拟表明,在气相和300 K的温度下,SO 2和H 2 O之间的主要相互作用为(SO 2) S··O (H 2 O),与先前在0 K下进行的密度泛函理论(DFT)计算一致。但是,在水纳米滴的表面,BOMD模拟表明(SO2) O···H(H 2 O)随着液滴尺寸的增加而变得越来越重要,这反映了水表面对SO 2溶剂化的显着影响。这一结论是根据先前获得的光谱证据良好( J.化学会志。 2005, 127,16806; J。化学会志。 2006, 128,3256)。大液滴上的主要相互作用(SO 2) O···H(H 2 O)主要归因于H 2的H原子的有利暴露在空气-水界面处为O。事实上,在气相中的主导相互作用的转化(SO 2) š...Ô (H 2 O)对水纳米液滴的主要相互作用(SO 2) Ô···ħ (H 2 O)可招致影响SO 2在大气中的气溶胶,因为SO 2在水表面的溶剂化会影响SO 2的反应位和亲电性。因此,当研究含气溶胶对流层中的SO 2化学时,气溶胶表面上的SO 2溶剂化可能具有新的含义。
更新日期:2017-11-16
中文翻译:

SO 2与水纳米液滴表面的相互作用
我们提出了一种SO 2与气相中的水分子以及与各种尺寸的水纳米液滴表面相互作用的综合计算研究,以研究SO 2在不同大气环境中的溶剂化行为。Born-Oppenheimer分子动力学(BOMD)模拟表明,在气相和300 K的温度下,SO 2和H 2 O之间的主要相互作用为(SO 2) S··O (H 2 O),与先前在0 K下进行的密度泛函理论(DFT)计算一致。但是,在水纳米滴的表面,BOMD模拟表明(SO2) O···H(H 2 O)随着液滴尺寸的增加而变得越来越重要,这反映了水表面对SO 2溶剂化的显着影响。这一结论是根据先前获得的光谱证据良好( J.化学会志。 2005, 127,16806; J。化学会志。 2006, 128,3256)。大液滴上的主要相互作用(SO 2) O···H(H 2 O)主要归因于H 2的H原子的有利暴露在空气-水界面处为O。事实上,在气相中的主导相互作用的转化(SO 2) š...Ô (H 2 O)对水纳米液滴的主要相互作用(SO 2) Ô···ħ (H 2 O)可招致影响SO 2在大气中的气溶胶,因为SO 2在水表面的溶剂化会影响SO 2的反应位和亲电性。因此,当研究含气溶胶对流层中的SO 2化学时,气溶胶表面上的SO 2溶剂化可能具有新的含义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号