当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption parameters and phase behaviour of non-ionic surfactants at liquid interfaces
Soft Matter ( IF 3.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7sm01370a Radomir Iliev Slavchov 1, 2, 3, 4 , Ivan Boyanov Ivanov 5, 6, 7, 8, 9
Soft Matter ( IF 3.4 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7sm01370a Radomir Iliev Slavchov 1, 2, 3, 4 , Ivan Boyanov Ivanov 5, 6, 7, 8, 9
Affiliation
|
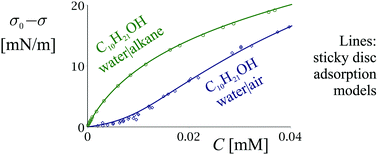
A reasonable adsorption model is one that allows all adsorption parameters (adsorption constant, hard-disc area α, attraction parameter β) of a surfactant at a liquid interface to be predicted accurately as a function of the molecular structure and medium conditions. However, the established adsorption models of van der Waals and Frumkin lead to inconsistencies, such as negative β at water|oil, α significantly larger than the crystallographic area of the molecule, and phase behaviour that contradicts the experimental observations. Several less popular models that are better suited for liquid interfaces are investigated. It is shown that the sticky disc model agrees with the observed adsorption behaviour of several homologous series of surfactants, both at water|air and water|oil interfaces. The area α is independent of the interface and agrees within 6% to what follows from collapse and crystallographic data. A model of the lateral attraction is proposed, from which it follows that β has a strongly non-linear dependence on the hydrocarbon chain length, the area of the head group and the temperature. Using the model of β, experimental data, and the law of corresponding states, the critical point of the adsorbed layer could be determined. Depending on the value of β, the adsorption behaviour of the surfactants at liquid interfaces can be classified into distinct categories: cohesive or non-cohesive, based on their Boyle points (where β = 2), and sub-critical or super-critical, based on their critical points (where β = 38.1).
中文翻译:

非离子表面活性剂在液体界面的吸附参数和相行为
合理的吸附模型是一种可以根据分子结构和介质条件准确预测液体界面上表面活性剂的所有吸附参数(吸附常数,硬盘面积α,吸引力参数β)的模型。然而,建立的范德华和弗鲁姆金的吸附模型导致不一致,例如在油处的负β,α明显大于该分子的晶体学面积,并且相行为与实验观察结果相矛盾。研究了几种较不适合用于液体界面的较不流行的模型。结果表明,粘性盘模型与在水和水界面上观察到的几种同源系列的表面活性剂的吸附行为一致。面积α与界面无关,并且与塌陷和晶体学数据相一致,在6%之内。提出了侧向吸引力的模型,由此得出,β对烃链长度,头基面积和温度具有很强的非线性依赖性。使用β模型,实验数据以及相应状态的定律,可以确定吸附层的临界点。根据β的值,表面活性剂在液体界面的吸附行为可以分为不同的类别:内聚性或非内聚性,基于它们的Boyle点(其中β = 2)以及次临界或超临界,根据他们的临界点(其中β = 38.1)。
更新日期:2017-11-16
中文翻译:

非离子表面活性剂在液体界面的吸附参数和相行为
合理的吸附模型是一种可以根据分子结构和介质条件准确预测液体界面上表面活性剂的所有吸附参数(吸附常数,硬盘面积α,吸引力参数β)的模型。然而,建立的范德华和弗鲁姆金的吸附模型导致不一致,例如在油处的负β,α明显大于该分子的晶体学面积,并且相行为与实验观察结果相矛盾。研究了几种较不适合用于液体界面的较不流行的模型。结果表明,粘性盘模型与在水和水界面上观察到的几种同源系列的表面活性剂的吸附行为一致。面积α与界面无关,并且与塌陷和晶体学数据相一致,在6%之内。提出了侧向吸引力的模型,由此得出,β对烃链长度,头基面积和温度具有很强的非线性依赖性。使用β模型,实验数据以及相应状态的定律,可以确定吸附层的临界点。根据β的值,表面活性剂在液体界面的吸附行为可以分为不同的类别:内聚性或非内聚性,基于它们的Boyle点(其中β = 2)以及次临界或超临界,根据他们的临界点(其中β = 38.1)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号