当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photocatalytic Oxidative Bromination of Electron-Rich Arenes and Heteroarenes by Anthraquinone
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-16 04:48:10 , DOI: 10.1002/adsc.201701276 Daniel Petzold 1 , Burkhard König 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-16 04:48:10 , DOI: 10.1002/adsc.201701276 Daniel Petzold 1 , Burkhard König 1
Affiliation
|
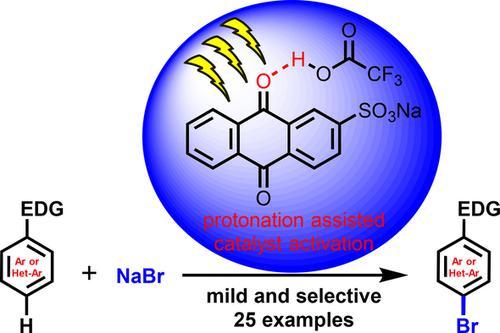
The estimated excited oxidation potential of sodium anthraquinone-2-sulfonate (SAS) increases from 1.8 V to about 2.3 V vs SCE by protonation with Brønsted acids. This increased photooxidation power of protonated anthraquinone was used for the regio-selective oxidative bromination of electron rich (hetero)arenes and drugs in good yield. The mild reaction conditions are compatible with many functional groups, such as double and triple bonds, ketones, amides and amines, hydroxyl groups, carboxylic acids and carbamates. Mechanistic investigations indicate the photooxidation of the arene followed by nucleophilic bromide addition as the likely pathway.
中文翻译:

蒽醌对富电子芳烃和杂芳烃的光催化氧化溴化反应
通过用布朗斯台德酸进行质子化,蒽醌-2-磺酸钠(SAS)的估计激发氧化电势相对于SCE从1.8 V增加到约2.3V。质子化蒽醌的这种增加的光氧化能力用于富电子(杂)芳烃和药物的区域选择性氧化溴化反应,收率很好。温和的反应条件与许多官能团兼容,例如双键和三键,酮,酰胺和胺,羟基,羧酸和氨基甲酸酯。机理研究表明,芳烃的光氧化反应继之以亲核性溴化物添加是可能的途径。
更新日期:2017-11-16
中文翻译:

蒽醌对富电子芳烃和杂芳烃的光催化氧化溴化反应
通过用布朗斯台德酸进行质子化,蒽醌-2-磺酸钠(SAS)的估计激发氧化电势相对于SCE从1.8 V增加到约2.3V。质子化蒽醌的这种增加的光氧化能力用于富电子(杂)芳烃和药物的区域选择性氧化溴化反应,收率很好。温和的反应条件与许多官能团兼容,例如双键和三键,酮,酰胺和胺,羟基,羧酸和氨基甲酸酯。机理研究表明,芳烃的光氧化反应继之以亲核性溴化物添加是可能的途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号