当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Propensity for cis-Proline Formation in Unfolded Proteins
ChemBioChem ( IF 3.2 ) Pub Date : 2017-11-16 09:30:34 , DOI: 10.1002/cbic.201700548 T. Reid Alderson 1 , Jung Ho Lee 1 , Cyril Charlier 1 , Jinfa Ying 1 , Ad Bax 1
ChemBioChem ( IF 3.2 ) Pub Date : 2017-11-16 09:30:34 , DOI: 10.1002/cbic.201700548 T. Reid Alderson 1 , Jung Ho Lee 1 , Cyril Charlier 1 , Jinfa Ying 1 , Ad Bax 1
Affiliation
|
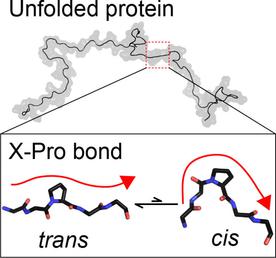
Quid Pro quo: Multidimensional NMR spectroscopy was used to quantify cis-Pro peptide bond fractions in three unfolded proteins. The cis values are systematically lower than those in the corresponding short peptides; this is attributed to reduced conformational entropy in the antiparallel segments preceding and following the cis-Pro peptide bonds and to electrostatic attraction between oppositely charged termini in unblocked peptides.
中文翻译:

折叠蛋白中顺式脯氨酸形成的倾向
Quid Pro:多维NMR光谱用于定量三种未折叠蛋白中的顺式-Pro肽键部分。的顺式值是系统地低于那些在相应的短肽; 这归因于顺式-Pro肽键之前和之后的反平行区段中构象熵的降低,以及未阻断肽中带相反电荷的末端之间的静电吸引。
更新日期:2017-11-16
中文翻译:

折叠蛋白中顺式脯氨酸形成的倾向
Quid Pro:多维NMR光谱用于定量三种未折叠蛋白中的顺式-Pro肽键部分。的顺式值是系统地低于那些在相应的短肽; 这归因于顺式-Pro肽键之前和之后的反平行区段中构象熵的降低,以及未阻断肽中带相反电荷的末端之间的静电吸引。


























 京公网安备 11010802027423号
京公网安备 11010802027423号