当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Below-Room-Temperature C–H Bond Breaking on an Inexpensive Metal Oxide: Methanol to Formaldehyde on CeO2(111)
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.jpclett.7b02683 Jonathan E. Sutton 1 , Thomas Danielson 2 , Ariana Beste 3 , Aditya Savara 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.jpclett.7b02683 Jonathan E. Sutton 1 , Thomas Danielson 2 , Ariana Beste 3 , Aditya Savara 1
Affiliation
|
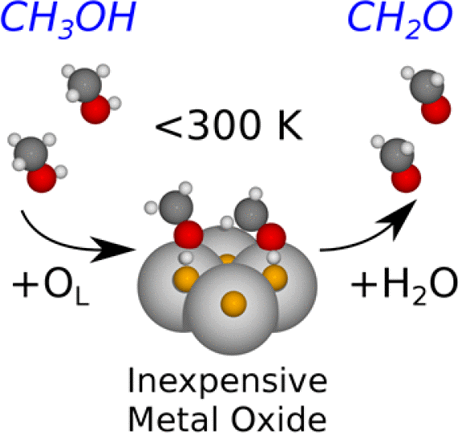
Upgrading of primary alcohols by C–H bond breaking currently requires temperatures of >200 °C. In this work, new understanding from simulation of a temperature-programmed reaction study with methanol over a CeO2(111) surface shows C–H bond breaking and the subsequent desorption of formaldehyde, even below room temperature. This is of particular interest because CeO2 is a naturally abundant and inexpensive metal oxide. We combine density functional theory and kinetic Monte Carlo methods to show that the low-temperature C–H bond breaking occurs via disproportionation of adjacent methoxy species. We further show from calculations that the same transition state with comparable activation energy exists for other primary alcohols; with ethanol, 1-propanol, and 1-butanol explicitly calculated. These findings indicate a promising class of transition states to search for in seeking low-temperature C–H bond breaking over inexpensive oxides.
中文翻译:

廉价金属氧化物上低于室温的C–H键断裂:CeO 2(111)上的甲醇至甲醛
目前,通过C–H键断裂来升级伯醇的温度要求> 200°C。在这项工作中,通过对甲醇在CeO 2(111)表面上进行程序升温反应研究的模拟获得的新认识表明,即使在室温以下,CH键断裂和甲醛的随后脱附也是如此。这是特别有趣的,因为CeO 2是一种天然丰富且廉价的金属氧化物。我们结合密度泛函理论和动力学蒙特卡罗方法来证明,低温C–H键断裂是通过相邻甲氧基种类的歧化而发生的。从计算中我们进一步表明,其他伯醇存在相同的过渡态,具有可比的活化能。用乙醇,1-丙醇和1-丁醇明确计算。这些发现表明,寻求廉价的氧化物打破低温C–H键的过渡态是有前途的。
更新日期:2017-11-16
中文翻译:

廉价金属氧化物上低于室温的C–H键断裂:CeO 2(111)上的甲醇至甲醛
目前,通过C–H键断裂来升级伯醇的温度要求> 200°C。在这项工作中,通过对甲醇在CeO 2(111)表面上进行程序升温反应研究的模拟获得的新认识表明,即使在室温以下,CH键断裂和甲醛的随后脱附也是如此。这是特别有趣的,因为CeO 2是一种天然丰富且廉价的金属氧化物。我们结合密度泛函理论和动力学蒙特卡罗方法来证明,低温C–H键断裂是通过相邻甲氧基种类的歧化而发生的。从计算中我们进一步表明,其他伯醇存在相同的过渡态,具有可比的活化能。用乙醇,1-丙醇和1-丁醇明确计算。这些发现表明,寻求廉价的氧化物打破低温C–H键的过渡态是有前途的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号