当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Iodine/Copper(I)-Catalyzed Direct Annulation of N-Benzimidazolyl Amidines with Aldehydes for the Synthesis of Ortho-Fused 1,3,5-Triazines
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-15 05:30:29 , DOI: 10.1002/adsc.201701126 Manman Wang 1 , Yinggao Meng 1 , Wei Wei 1 , Jie Wu 1 , Wenquan Yu 1 , Junbiao Chang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2017-11-15 05:30:29 , DOI: 10.1002/adsc.201701126 Manman Wang 1 , Yinggao Meng 1 , Wei Wei 1 , Jie Wu 1 , Wenquan Yu 1 , Junbiao Chang 1
Affiliation
|
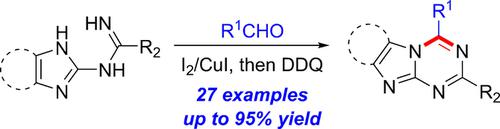
A direct annulation reaction of N-benzimidazolyl amidines with aldehydes has been established and allows the synthesis of ortho-fused 1,3,5-triazine derivatives. The N-benzimidazolyl amidine substrates are readily accessible by the addition of 2-aminobenzimidazoles to the corresponding nitriles. In the presence of molecular iodine and copper iodide, cyclization of benzimidazolyl amidines with various aldehydes in toluene under reflux followed by oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) gives the corresponding products. In this reaction, iodine acts more as a Lewis acid catalyst than as an oxidant. This synthetic process is insensitive to air and operationally simple, and provides facile access to a variety of novel 1,3,5-triazino[1,2-a]benzimidazoles and related heterocycles.
中文翻译:

碘/铜(I)催化的N-苯并咪唑基Am与醛的环化反应,用于邻位融合的1,3,5-三嗪的合成
已经建立了N-苯并咪唑基am与醛的直接环化反应,并允许合成邻位熔融的1,3,5-三嗪衍生物。所述Ñ -benzimidazolyl脒底物是通过加入2-氨基苯并咪唑,以相应的腈容易接近。在分子碘和碘化铜的存在下,苯并咪唑基am与甲苯中的各种醛在回流下环化,然后用2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)氧化,得到相应的产物。在该反应中,碘起路易斯酸催化剂的作用比起氧化剂起更多的作用。这种合成工艺对空气不敏感,操作简单,可轻松接触各种新颖的1,3,5-三嗪[1,2-a ]苯并咪唑及相关杂环。
更新日期:2017-11-16
中文翻译:

碘/铜(I)催化的N-苯并咪唑基Am与醛的环化反应,用于邻位融合的1,3,5-三嗪的合成
已经建立了N-苯并咪唑基am与醛的直接环化反应,并允许合成邻位熔融的1,3,5-三嗪衍生物。所述Ñ -benzimidazolyl脒底物是通过加入2-氨基苯并咪唑,以相应的腈容易接近。在分子碘和碘化铜的存在下,苯并咪唑基am与甲苯中的各种醛在回流下环化,然后用2,3-二氯-5,6-二氰基-1,4-苯醌(DDQ)氧化,得到相应的产物。在该反应中,碘起路易斯酸催化剂的作用比起氧化剂起更多的作用。这种合成工艺对空气不敏感,操作简单,可轻松接触各种新颖的1,3,5-三嗪[1,2-a ]苯并咪唑及相关杂环。



























 京公网安备 11010802027423号
京公网安备 11010802027423号