当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Infrared Spectroscopic and Electronic Structure Investigations of Beryllium Halide Molecules, Cations, and Anions in Noble Gas Matrices
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.jpca.7b09454 Wenjie Yu 1 , Lester Andrews 2 , Xuefeng Wang 1, 2
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.jpca.7b09454 Wenjie Yu 1 , Lester Andrews 2 , Xuefeng Wang 1, 2
Affiliation
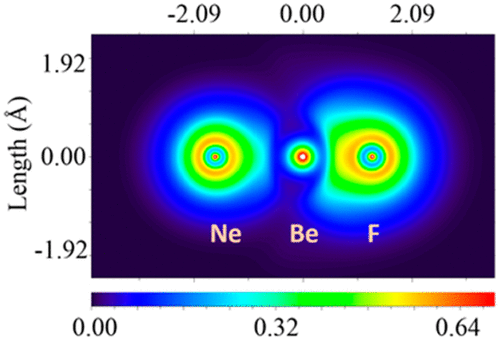
|
Laser-ablated Be atoms, cations, and electrons were reacted with F2, ClF, Cl2, NF3, CCl4, CF2Cl2, HCl, DCl, and SiCl4 diluted in noble gases. The major products were the dihalides BeF2, BeClF, BeCl2, and the hydride chloride HBeCl, whose identities were confirmed by comparison with previous evaporative work, deuterium substitution, and vibrational frequency calculations. The matrix-isolated fundamental frequency of the BeF molecule is higher, and the frequency of BeCl is lower, than that determined for the gas-phase molecules. The BeF+ and BeCl+ cations formed strong dipole-induced dipole complexes in solid Ne, Ar, Kr, and Xe with stepwise increase in computed noble gas dissociation energies. Going down the family NgBeF+ and NgBeCl+ series (Ng = Ne, Ar, Kr, Xe) the Mulliken charges q(Be) decrease, while q(Ng) increases, and the dipole moments decrease, which suggests covalent bonding in the xenon species. We find that the largest intramatrix shift is Ne to Ar which follows the largest factor increase for the Ng atomic polarizabilities. Extra electrons produce Cl–, which reacts with HCl to form the stable HCl2– anion and possibly with BeCl2 to give BeCl3–. A weak band observed in neon experiments with F2 is probably due to BeF3–.
中文翻译:

惰性气体基质中卤化铍分子,阳离子和阴离子的红外光谱和电子结构研究
激光烧蚀的Be原子,阳离子和电子与稀有气体中稀释的F 2,ClF,Cl 2,NF 3,CCl 4,CF 2 Cl 2,HCl,DCl和SiCl 4反应。主要产品是二卤化物BeF 2,BeClF,BeCl 2和氯化氢HBeCl,它们的身份通过与之前的蒸发工作,氘代和振动频率计算的比较得到证实。与气相分子确定的相比,BeF分子在基质上隔离的基本频率更高,而BeCl的频率更低。BeF +和BeCl +阳离子在固体Ne,Ar,Kr和Xe中形成强偶极子诱导的偶极子配合物,计算的稀有气体离解能逐步增加。沿着NgBeF +和NgBeCl +系列(Ng = Ne,Ar,Kr,Xe),Mulliken电荷q(Be)减小,而q(Ng)增大,偶极矩减小,这表明氙中的共价键物种。我们发现最大的矩阵内位移是Ne到Ar,这是Ng原子极化率增加最大的因素。额外的电子产生氯- ,用HCl反应以形成稳定的HCl这2 -阴离子和可能与BECL 2,得到BECL 3 -。在氖实验中观察到与F A弱带2可能是由于BEF 3 - 。
更新日期:2017-11-16
中文翻译:

惰性气体基质中卤化铍分子,阳离子和阴离子的红外光谱和电子结构研究
激光烧蚀的Be原子,阳离子和电子与稀有气体中稀释的F 2,ClF,Cl 2,NF 3,CCl 4,CF 2 Cl 2,HCl,DCl和SiCl 4反应。主要产品是二卤化物BeF 2,BeClF,BeCl 2和氯化氢HBeCl,它们的身份通过与之前的蒸发工作,氘代和振动频率计算的比较得到证实。与气相分子确定的相比,BeF分子在基质上隔离的基本频率更高,而BeCl的频率更低。BeF +和BeCl +阳离子在固体Ne,Ar,Kr和Xe中形成强偶极子诱导的偶极子配合物,计算的稀有气体离解能逐步增加。沿着NgBeF +和NgBeCl +系列(Ng = Ne,Ar,Kr,Xe),Mulliken电荷q(Be)减小,而q(Ng)增大,偶极矩减小,这表明氙中的共价键物种。我们发现最大的矩阵内位移是Ne到Ar,这是Ng原子极化率增加最大的因素。额外的电子产生氯- ,用HCl反应以形成稳定的HCl这2 -阴离子和可能与BECL 2,得到BECL 3 -。在氖实验中观察到与F A弱带2可能是由于BEF 3 - 。



























 京公网安备 11010802027423号
京公网安备 11010802027423号