当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of the Membrane Binding Domain in Trifunctional Proline Utilization A
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.biochem.7b01008 Shelbi L. Christgen 1 , Weidong Zhu 1 , Nikhilesh Sanyal 1 , Bushra Bibi 1 , John J. Tanner , Donald F. Becker 1
Biochemistry ( IF 2.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.biochem.7b01008 Shelbi L. Christgen 1 , Weidong Zhu 1 , Nikhilesh Sanyal 1 , Bushra Bibi 1 , John J. Tanner , Donald F. Becker 1
Affiliation
|
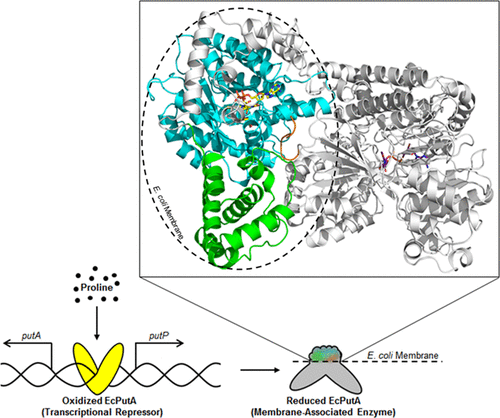
Escherichia coli proline utilization A (EcPutA) is the archetype of trifunctional PutA flavoproteins, which function both as regulators of the proline utilization operon and bifunctional enzymes that catalyze the four-electron oxidation of proline to glutamate. EcPutA shifts from a self-regulating transcriptional repressor to a bifunctional enzyme in a process known as functional switching. The flavin redox state dictates the function of EcPutA. Upon proline oxidation, the flavin becomes reduced, triggering a conformational change that causes EcPutA to dissociate from the put regulon and bind to the cellular membrane. Major structure/function domains of EcPutA have been characterized, including the DNA-binding domain, proline dehydrogenase (PRODH) and l-glutamate-γ-semialdehyde dehydrogenase catalytic domains, and an aldehyde dehydrogenase superfamily fold domain. Still lacking is an understanding of the membrane-binding domain, which is essential for EcPutA catalytic turnover and functional switching. Here, we provide evidence for a conserved C-terminal motif (CCM) in EcPutA having a critical role in membrane binding. Deletion of the CCM or replacement of hydrophobic residues with negatively charged residues within the CCM impairs EcPutA functional and physical membrane association. Furthermore, cell-based transcription assays and limited proteolysis indicate that the CCM is essential for functional switching. Using fluorescence resonance energy transfer involving dansyl-labeled liposomes, residues in the α-domain are also implicated in membrane binding. Taken together, these experiments suggest that the CCM and α-domain converge to form a membrane-binding interface near the PRODH domain. The discovery of the membrane-binding region will assist efforts to define flavin redox signaling pathways responsible for EcPutA functional switching.
中文翻译:

在三功能脯氨酸利用中发现膜结合域
大肠杆菌脯氨酸利用A(Ec PutA)是三功能PutA黄素蛋白的原型,它既是脯氨酸利用操纵子的调节剂,又是催化脯氨酸四电子氧化为谷氨酸的双功能酶。Ec PutA在称为功能切换的过程中从自我调节的转录阻遏物转变为双功能酶。黄素氧化还原状态决定了Ec PutA的功能。脯氨酸氧化后,黄素还原,触发构象变化,导致Ec PutA从put regulon解离并结合到细胞膜上。Ec的主要结构/功能域PutA已经被表征,包括DNA结合结构域,脯氨酸脱氢酶(PRODH)和1-谷氨酸-γ-半醛脱氢酶催化结构域以及醛脱氢酶超家族折叠结构域。仍然缺乏对膜结合结构域的理解,这对于Ec PutA催化转换和功能转换至关重要。在这里,我们为Ec PutA中的C端保守基序(CCM)在膜结合中起关键作用提供了证据。删除CCM或用CCM中带负电荷的残基替换疏水残基会损害EcPutA功能和物理膜的关联。此外,基于细胞的转录测定法和有限的蛋白水解作用表明CCM对于功能转换至关重要。使用涉及丹磺酰基标记的脂质体的荧光共振能量转移,α-结构域中的残基也与膜结合有关。综上所述,这些实验表明CCM和α结构域会聚,在PRODH结构域附近形成膜结合界面。膜结合区的发现将有助于确定负责Ec PutA功能转换的黄素氧化还原信号通路。
更新日期:2017-11-16
中文翻译:

在三功能脯氨酸利用中发现膜结合域
大肠杆菌脯氨酸利用A(Ec PutA)是三功能PutA黄素蛋白的原型,它既是脯氨酸利用操纵子的调节剂,又是催化脯氨酸四电子氧化为谷氨酸的双功能酶。Ec PutA在称为功能切换的过程中从自我调节的转录阻遏物转变为双功能酶。黄素氧化还原状态决定了Ec PutA的功能。脯氨酸氧化后,黄素还原,触发构象变化,导致Ec PutA从put regulon解离并结合到细胞膜上。Ec的主要结构/功能域PutA已经被表征,包括DNA结合结构域,脯氨酸脱氢酶(PRODH)和1-谷氨酸-γ-半醛脱氢酶催化结构域以及醛脱氢酶超家族折叠结构域。仍然缺乏对膜结合结构域的理解,这对于Ec PutA催化转换和功能转换至关重要。在这里,我们为Ec PutA中的C端保守基序(CCM)在膜结合中起关键作用提供了证据。删除CCM或用CCM中带负电荷的残基替换疏水残基会损害EcPutA功能和物理膜的关联。此外,基于细胞的转录测定法和有限的蛋白水解作用表明CCM对于功能转换至关重要。使用涉及丹磺酰基标记的脂质体的荧光共振能量转移,α-结构域中的残基也与膜结合有关。综上所述,这些实验表明CCM和α结构域会聚,在PRODH结构域附近形成膜结合界面。膜结合区的发现将有助于确定负责Ec PutA功能转换的黄素氧化还原信号通路。



























 京公网安备 11010802027423号
京公网安备 11010802027423号