当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tm-Values and Unfolded Fraction Can Predict Aggregation Rates for Granulocyte Colony Stimulating Factor Variant Formulations but Not under Predominantly Native Conditions
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00876 Mathew J. Robinson 1 , Paul Matejtschuk 2 , Adrian F. Bristow 2 , Paul A. Dalby 1
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2017-11-28 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00876 Mathew J. Robinson 1 , Paul Matejtschuk 2 , Adrian F. Bristow 2 , Paul A. Dalby 1
Affiliation
|
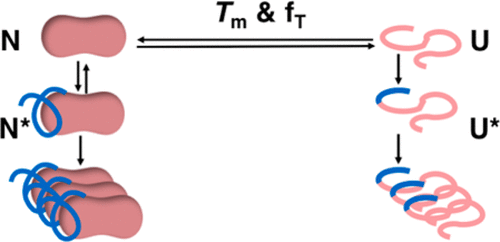
Protein engineering and formulation optimization strategies can be taken to minimize protein aggregation in the biopharmaceutical industry. Short-term stability measures such as the midpoint transition temperature (Tm) for global unfolding provide convenient surrogates for longer-term (e.g., 2-year) degradation kinetics, with which to optimize formulations on practical time-scales. While successful in some cases, their limitations have not been fully evaluated or understood. Tm values are known to correlate with chemical degradation kinetics for wild-type granulocyte colony stimulating factor (GCSF) at pH 4–5.5. However, we found previously that the Tm of an antibody Fab fragment only correlated with its rate of monomer loss at temperatures close to the Tm. Here we evaluated Tm, the fraction of unfolded protein (fT) at temperature T, and two additional short-term stability measures, for their ability to predict the kinetics of monomer and bioactivity loss of wild-type GCSF and four variants, at 37 °C, and in a wide range of formulations. The GCSF variants introduced one to three mutations, giving a range of conformational stabilities spanning 7.8 kcal mol–1. We determined the extent to which the formulation rank order differs across the variants when evaluated by each of the four short-term stability measures. All correlations decreased as the difference in average Tm between each pair of GCSF variants increased. The rank order of formulations determined by Tm was the best preserved, with R2-values >0.7. Tm-values also provided a good predictor (R2 = 0.73) of the aggregation rates, extending previous findings to include GCSF variant-formulation combinations. Further analysis revealed that GCSF aggregation rates at 37 °C were dependent on the fraction unfolded at 37 °C (fT37), but transitioned smoothly to a constant baseline rate of aggregation at fT37 < 10–3. A similar function was observed previously for A33 Fab formulated by pH, ionic strength, and temperature, without excipients. For GCSF, all combinations of variants and formulations fit onto a single curve, suggesting that even single mutations destabilized by up to 4.8 kcal mol–1, are insufficient to change significantly the baseline rate of aggregation under native conditions. The baseline rate of aggregation for GCSF under native conditions was 66-fold higher than that for A33 Fab, highlighting that they are a specific feature of each native protein structure, likely to be dependent on local surface properties and dynamics.
中文翻译:

T m值和未折叠的分数可以预测粒细胞集落刺激因子变异制剂的聚集率,但在天然条件下却不如此
可以采用蛋白质工程和配方优化策略来最大程度地减少生物制药行业中的蛋白质聚集。短期稳定性度量(例如用于全局展开的中点转变温度(T m))为长期(例如2年)降解动力学提供了方便的替代方法,从而可以在实际时标上优化配方。尽管在某些情况下是成功的,但尚未完全评估或理解其局限性。已知T m值与pH 4-5.5的野生型粒细胞集落刺激因子(GCSF)的化学降解动力学相关。但是,我们先前发现T m抗体Fab片段的分子量仅与在接近T m的温度下单体丢失的速率有关。在这里,我们评估了T m,在温度T时未折叠的蛋白质(f T)的比例以及两个其他的短期稳定性指标,它们能够预测野生型GCSF及其四个变体的单体动力学和生物活性损失。 37°C,适用于各种配方。GCSF变体引入了一到三个突变,从而提供了一系列构象稳定性,范围横跨7.8 kcal mol –1。当通过四个短期稳定性指标中的每一个进行评估时,我们确定了制剂等级顺序在各个变体之间的差异程度。随着每对GCSF变体之间平均T m的差异增加,所有相关性均下降。由T m确定的制剂的等级顺序保存得最好,R 2值> 0.7。T m值还提供了聚集率的良好预测指标(R 2 = 0.73),将先前的发现扩展到包括GCSF变体-配方组合。进一步的分析表明,在37°C下GCSF的聚集速率取决于在37°C下展开的分数(fT37),但在f T37 <10 –3时平稳过渡到恒定的基线聚集速率。以前在没有赋形剂的情况下,通过pH,离子强度和温度配制的A33 Fab观察到了相似的功能。对于GCSF,变体和配方的所有组合都拟合在一条曲线上,这表明即使单个突变被高达4.8 kcal mol –1破坏了稳定性,也不足以显着改变天然条件下的基线聚集速率。在自然条件下,GCSF的基线聚集速率比A33 Fab的基线聚集速率高66倍,这表明它们是每种天然蛋白质结构的特定特征,可能取决于局部表面特性和动力学。
更新日期:2017-11-28
中文翻译:

T m值和未折叠的分数可以预测粒细胞集落刺激因子变异制剂的聚集率,但在天然条件下却不如此
可以采用蛋白质工程和配方优化策略来最大程度地减少生物制药行业中的蛋白质聚集。短期稳定性度量(例如用于全局展开的中点转变温度(T m))为长期(例如2年)降解动力学提供了方便的替代方法,从而可以在实际时标上优化配方。尽管在某些情况下是成功的,但尚未完全评估或理解其局限性。已知T m值与pH 4-5.5的野生型粒细胞集落刺激因子(GCSF)的化学降解动力学相关。但是,我们先前发现T m抗体Fab片段的分子量仅与在接近T m的温度下单体丢失的速率有关。在这里,我们评估了T m,在温度T时未折叠的蛋白质(f T)的比例以及两个其他的短期稳定性指标,它们能够预测野生型GCSF及其四个变体的单体动力学和生物活性损失。 37°C,适用于各种配方。GCSF变体引入了一到三个突变,从而提供了一系列构象稳定性,范围横跨7.8 kcal mol –1。当通过四个短期稳定性指标中的每一个进行评估时,我们确定了制剂等级顺序在各个变体之间的差异程度。随着每对GCSF变体之间平均T m的差异增加,所有相关性均下降。由T m确定的制剂的等级顺序保存得最好,R 2值> 0.7。T m值还提供了聚集率的良好预测指标(R 2 = 0.73),将先前的发现扩展到包括GCSF变体-配方组合。进一步的分析表明,在37°C下GCSF的聚集速率取决于在37°C下展开的分数(fT37),但在f T37 <10 –3时平稳过渡到恒定的基线聚集速率。以前在没有赋形剂的情况下,通过pH,离子强度和温度配制的A33 Fab观察到了相似的功能。对于GCSF,变体和配方的所有组合都拟合在一条曲线上,这表明即使单个突变被高达4.8 kcal mol –1破坏了稳定性,也不足以显着改变天然条件下的基线聚集速率。在自然条件下,GCSF的基线聚集速率比A33 Fab的基线聚集速率高66倍,这表明它们是每种天然蛋白质结构的特定特征,可能取决于局部表面特性和动力学。



























 京公网安备 11010802027423号
京公网安备 11010802027423号