Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of Tail Groups during Functionalization of ZnO Nanoparticles on Binding Enthalpies and Photoluminescence
Langmuir ( IF 3.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.langmuir.7b03079 Wei Lin 1, 2 , Jochen Schmidt 1, 2 , Michael Mahler 1 , Torben Schindler 3 , Tobias Unruh 3 , Bernd Meyer 4 , Wolfgang Peukert 1, 2 , Doris Segets 1, 2
Langmuir ( IF 3.9 ) Pub Date : 2017-11-15 00:00:00 , DOI: 10.1021/acs.langmuir.7b03079 Wei Lin 1, 2 , Jochen Schmidt 1, 2 , Michael Mahler 1 , Torben Schindler 3 , Tobias Unruh 3 , Bernd Meyer 4 , Wolfgang Peukert 1, 2 , Doris Segets 1, 2
Affiliation
|
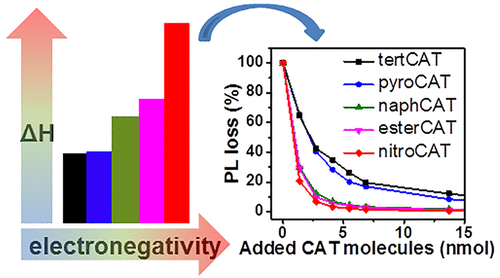
We report on the tailoring of ZnO nanoparticle (NP) surfaces by catechol derivatives (CAT) with different functionalities: tert-butyl group (tertCAT), hydrogen (pyroCAT), aromatic ring (naphCAT), ester group (esterCAT), and nitro group (nitroCAT). The influence of electron-donating/-withdrawing properties on enthalpy of ligand binding (ΔH) was resolved and subsequently linked with optical properties. First, as confirmed by ultraviolet/visible (UV/vis) and Fourier transform infrared (FT-IR) spectroscopy results, all CAT molecules chemisorbed to ZnO NPs, independent of the distinct functionality. Interestingly, the ζ-potentials of ZnO after functionalization shifted to more negative values. Then, isothermal titration calorimetry (ITC) and a mass-based method were applied to resolve the heat release during ligand binding and the adsorption isotherm, respectively. However, both heat- and mass-based approaches alone did not fully resolve the binding enthalpy of each molecule adsorbing to the ZnO surface. This is mainly due to the fact that the Langmuir model oversimplifies the underlying adsorption mechanism, at least for some of the tested CAT molecules. Therefore, a new, fitting-free approach was developed to directly access the adsorption enthalpy per molecule during functionalization by dividing the heat release measured via ITC by the amount of bound molecules determined from the adsorption isotherm. Finally, the efficiency of quenching the visible emission caused by ligand binding was investigated by photoluminescence (PL) spectroscopy, which turned out to follow the same trend as the binding enthalpy. Thus, the functionality of ligand molecules governs the binding enthalpy to the particle surface, which in turn, at least in the current case of ZnO, is an important parameter for the quenching of visible emission. We believe that establishing such correlations is an important step toward a more general way of selecting and designing ligand molecules for surface functionalization. This allows developing strategies for tailored colloidal surfaces beyond empirically driven formulation on a case by case basis.
中文翻译:

ZnO纳米粒子功能化过程中尾基对结合焓和光致发光的影响
我们报告了具有不同功能的邻苯二酚衍生物(CAT)对ZnO纳米颗粒(NP)表面的定制:叔丁基(tertCAT),氢(pyroCAT),芳环(naphCAT),酯基(esterCAT)和硝基(nitroCAT)。给电子/吸电子性能对配体结合焓的影响(ΔH)被解析,并随后与光学性质相关联。首先,通过紫外/可见(UV / vis)和傅立叶变换红外(FT-IR)光谱学结果证实,所有CAT分子均化学吸附到ZnO NP上,而与独特的功能无关。有趣的是,官能化后的ZnO的ζ电位转变为更多的负值。然后,采用等温滴定热法(ITC)和基于质量的方法分别解决配体结合和吸附等温线过程中的放热问题。然而,仅基于热和基于质量的方法都不能完全解决吸附到ZnO表面的每个分子的结合焓。这主要是由于Langmuir模型过分简化了潜在的吸附机制,至少对于某些测试的CAT分子而言如此。因此,一个新的,通过采用ITC测量的放热除以由吸附等温线确定的结合分子的量,开发了无拟合方法,可在功能化过程中直接访问每个分子的吸附焓。最后,通过光致发光(PL)光谱研究了猝灭由配体结合引起的可见光发射的效率,结果证明其遵循与结合焓相同的趋势。因此,配体分子的功能性决定了与颗粒表面的结合焓,这至少在当前的ZnO情况下是可见光猝灭的重要参数。我们相信,建立这种相关性是朝着选择和设计用于表面功能化的配体分子的更通用方法迈出的重要一步。
更新日期:2017-11-15
中文翻译:

ZnO纳米粒子功能化过程中尾基对结合焓和光致发光的影响
我们报告了具有不同功能的邻苯二酚衍生物(CAT)对ZnO纳米颗粒(NP)表面的定制:叔丁基(tertCAT),氢(pyroCAT),芳环(naphCAT),酯基(esterCAT)和硝基(nitroCAT)。给电子/吸电子性能对配体结合焓的影响(ΔH)被解析,并随后与光学性质相关联。首先,通过紫外/可见(UV / vis)和傅立叶变换红外(FT-IR)光谱学结果证实,所有CAT分子均化学吸附到ZnO NP上,而与独特的功能无关。有趣的是,官能化后的ZnO的ζ电位转变为更多的负值。然后,采用等温滴定热法(ITC)和基于质量的方法分别解决配体结合和吸附等温线过程中的放热问题。然而,仅基于热和基于质量的方法都不能完全解决吸附到ZnO表面的每个分子的结合焓。这主要是由于Langmuir模型过分简化了潜在的吸附机制,至少对于某些测试的CAT分子而言如此。因此,一个新的,通过采用ITC测量的放热除以由吸附等温线确定的结合分子的量,开发了无拟合方法,可在功能化过程中直接访问每个分子的吸附焓。最后,通过光致发光(PL)光谱研究了猝灭由配体结合引起的可见光发射的效率,结果证明其遵循与结合焓相同的趋势。因此,配体分子的功能性决定了与颗粒表面的结合焓,这至少在当前的ZnO情况下是可见光猝灭的重要参数。我们相信,建立这种相关性是朝着选择和设计用于表面功能化的配体分子的更通用方法迈出的重要一步。


























 京公网安备 11010802027423号
京公网安备 11010802027423号