PLOS Medicine ( IF 15.8 ) Pub Date : 2017-11-14 , DOI: 10.1371/journal.pmed.1002428 Rita R. Alloway , Alexander A. Vinks , Tsuyoshi Fukuda , Tomoyuki Mizuno , Eileen C. King , Yuanshu Zou , Wenlei Jiang , E. Steve Woodle , Simon Tremblay , Jelena Klawitter , Jost Klawitter , Uwe Christians
|
Background
Although the generic drug approval process has a long-term successful track record, concerns remain for approval of narrow therapeutic index generic immunosuppressants, such as tacrolimus, in transplant recipients. Several professional transplant societies and publications have generated skepticism of the generic approval process. Three major areas of concern are that the pharmacokinetic properties of generic products and the innovator (that is, “brand”) product in healthy volunteers may not reflect those in transplant recipients, bioequivalence between generic and innovator may not ensure bioequivalence between generics, and high-risk patients may have specific bioequivalence concerns. Such concerns have been fueled by anecdotal observations and retrospective and uncontrolled published studies, while well-designed, controlled prospective studies testing the validity of the regulatory bioequivalence testing approach for narrow therapeutic index immunosuppressants in transplant recipients have been lacking. Thus, the present study prospectively assesses bioequivalence between innovator tacrolimus and 2 generics in individuals with a kidney or liver transplant.
Methods and findings
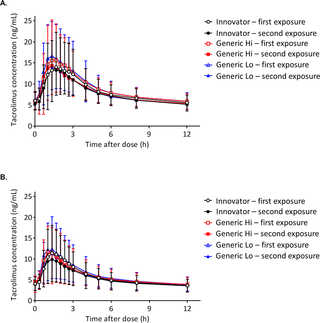
From December 2013 through October 2014, a prospective, replicate dosing, partially blinded, randomized, 3-treatment, 6-period crossover bioequivalence study was conducted at the University of Cincinnati in individuals with a kidney (n = 35) or liver transplant (n = 36). Abbreviated New Drug Applications (ANDA) data that included manufacturing and healthy individual pharmacokinetic data for all generics were evaluated to select the 2 most disparate generics from innovator, and these were named Generic Hi and Generic Lo. During the 8-week study period, pharmacokinetic studies assessed the bioequivalence of Generic Hi and Generic Lo with the Innovator tacrolimus and with each other. Bioequivalence of the major tacrolimus metabolite was also assessed. All products fell within the US Food and Drug Administration (FDA) average bioequivalence (ABE) acceptance criteria of a 90% confidence interval contained within the confidence limits of 80.00% and 125.00%. Within-subject variability was similar for the area under the curve (AUC) (range 12.11–15.81) and the concentration maximum (Cmax) (range 17.96–24.72) for all products. The within-subject variability was utilized to calculate the scaled average bioequivalence (SCABE) 90% confidence interval. The calculated SCABE 90% confidence interval was 84.65%–118.13% and 80.00%–125.00% for AUC and Cmax, respectively. The more stringent SCABE acceptance criteria were met for all product comparisons for AUC and Cmax in both individuals with a kidney transplant and those with a liver transplant. European Medicines Agency (EMA) acceptance criteria for narrow therapeutic index drugs were also met, with the only exception being in the case of Brand versus Generic Lo, in which the upper limits of the 90% confidence intervals were 111.30% (kidney) and 112.12% (liver). These were only slightly above the upper EMA acceptance criteria limit for an AUC of 111.11%. SCABE criteria were also met for the major tacrolimus metabolite 13-O-desmethyl tacrolimus for AUC, but it failed the EMA criterion. No acute rejections, no differences in renal function in all individuals, and no differences in liver function were observed in individuals with a liver transplant using the Tukey honest significant difference (HSD) test for multiple comparisons. Fifty-two percent and 65% of all individuals with a kidney or liver transplant, respectively, reported an adverse event. The Exact McNemar test for paired categorical data with adjustments for multiple comparisons was used to compare adverse event rates among the products. No statistically significant differences among any pairs of products were found for any adverse event code or for adverse events overall. Limitations of this study include that the observations were made under strictly controlled conditions that did not allow for the impact of nonadherence or feeding on the possible pharmacokinetic differences. Generic Hi and Lo were selected based upon bioequivalence data in healthy volunteers because no pharmacokinetic data in recipients were available for all products. The safety data should be interpreted in light of the small number of participants and the short observation periods. Lastly, only the 1 mg tacrolimus strength was utilized in this study.
Conclusions
Using an innovative, controlled bioequivalence study design, we observed equivalence between tacrolimus innovator and 2 generic products as well as between 2 generic products in individuals after kidney or liver transplantation following current FDA bioequivalence metrics. These results support the position that bioequivalence for the narrow therapeutic index drug tacrolimus translates from healthy volunteers to individuals receiving a kidney or liver transplant and provides evidence that generic products that are bioequivalent with the innovator product are also bioequivalent to each other.
Trial registration
ClinicalTrials.gov NCT01889758.
中文翻译:

肝肾移植受者中创新者与通用他克莫司之间的生物等效性:一项随机,交叉的临床试验
背景
尽管仿制药批准过程具有长期成功的记录,但仍需要在移植受体中批准窄治疗指数仿制药免疫抑制剂(如他克莫司)。几个专业的移植协会和出版物对通用批准程序产生了怀疑。关注的三个主要领域是,健康志愿者中的仿制药和创新者(即“品牌”)产品的药代动力学特性可能无法反映移植受者的药代动力学特性,仿制药与创新者之间的生物等效性可能无法确保仿制药与生物等效性之间的生物等效性。高危患者可能有特定的生物等效性问题。尽管经过精心设计,但轶事性观察,回顾性和不受控制的已发表研究都加剧了这种担忧,缺乏针对移植受者中狭窄治疗指数免疫抑制剂的调节性生物等效性测试方法的有效性的对照前瞻性研究。因此,本研究前瞻性地评估了肾脏或肝脏移植患者中他克莫司和2种仿制药之间的生物等效性。
方法和发现
从2013年12月至2014年10月,在辛辛那提大学针对肾脏(n = 35)或肝移植(n= 36)。评估包括所有仿制药的制造和健康个体药代动力学数据的简明新药申请(ANDA)数据,以选择创新者中2种最不相同的仿制药,并将它们命名为Generic Hi和Generic Lo。在为期8周的研究期内,药代动力学研究评估了创新型他克莫司和彼此之间Generic Hi和Generic Lo的生物等效性。还评估了他克莫司主要代谢物的生物等效性。所有产品均符合90%置信区间的美国食品和药物管理局(FDA)平均生物等效性(ABE)接受标准,其置信度范围为80.00%和125.00%。曲线下面积(AUC)(范围12.11–15.81)和最大浓度(C max)的受试者内变异性相似)(范围为17.96–24.72)以用于所有产品。利用受试者内部的变异性计算比例平均生物等效性(SCABE)90%置信区间。对于AUC和C max,计算得出的SCABE 90%置信区间分别为84.65%–118.13%和80.00%–125.00%。所有产品对AUC和C max的比较均符合更严格的SCABE接受标准在有肾移植和有肝移植的两个人中。还满足了欧洲药品管理局(EMA)对窄治疗指数药物的接受标准,但唯一的例外是Brand与Generic Lo,其90%置信区间的上限为111.30%(肾脏)和112.12 % (肝脏)。这些仅略高于EMA接受标准的上限(AUC为111.11%)。AUC的主要他克莫司代谢产物13-O-去甲基他克莫司也符合SCABE标准,但未通过EMA标准。使用Tukey诚实显着性差异(HSD)检验进行多重比较,在所有肝移植患者中均未观察到急性排斥反应,所有患者的肾功能均未见差异以及肝功能均未见差异。分别有肾移植和肝移植的所有个体中有52%和65%报告了不良事件。使用精确的McNemar检验对配对的分类数据进行多次比较调整,以比较产品之间的不良事件发生率。在任何不良事件代码或整体不良事件中,在任何产品对之间均未发现统计学上的显着差异。该研究的局限性在于观察是在严格控制的条件下进行的,该条件不允许不依从或进食对可能的药代动力学差异的影响。根据健康志愿者的生物等效性数据选择通用的Hi和Lo,因为没有针对所有产品的接受者的药代动力学数据。应根据参加者人数少和观察期短的原因来解释安全数据。最后,在这项研究中仅使用了1 mg他克莫司强度。
结论
使用创新的,受控的生物等效性研究设计,我们观察了他克莫司创新剂和2种仿制药之间的等效性,以及按照现行FDA生物等效性指标在肾脏或肝脏移植后个体中2种仿制药之间的等效性。这些结果支持以下观点:窄治疗指数药物他克莫司的生物等效性从健康志愿者转化为接受肾脏或肝脏移植的个体,并提供了与创新产品生物等效的通用产品彼此生物等效的证据。
试用注册
ClinicalTrials.gov NCT01889758。


























 京公网安备 11010802027423号
京公网安备 11010802027423号