当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ergodicity breaking of iron displacement in heme proteins
Soft Matter ( IF 3.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7sm01561e Salman Seyedi 1, 2, 3, 4 , Dmitry V. Matyushov 2, 3, 4, 5
Soft Matter ( IF 3.4 ) Pub Date : 2017-10-23 00:00:00 , DOI: 10.1039/c7sm01561e Salman Seyedi 1, 2, 3, 4 , Dmitry V. Matyushov 2, 3, 4, 5
Affiliation
|
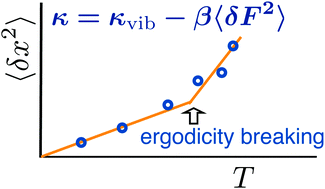
We present a model of the dynamical transition of atomic displacements in proteins. Increased mean-square displacement at higher temperatures is caused by the softening of the force constant for atomic/molecular displacements by electrostatic and van der Waals forces from the protein–water thermal bath. Displacement softening passes through a nonergodic dynamical transition when the relaxation time of the force–force correlation function enters, with increasing temperature, the instrumental observation window. Two crossover temperatures are identified. The lower crossover, presently connected to the glass transition, is related to the dynamical unfreezing of rotations of water molecules within nanodomains polarized by charged surface residues of the protein. The higher crossover temperature, usually assigned to the dynamical transition, marks the onset of water translations. All crossovers are ergodicity breaking transitions depending on the corresponding observation windows. Allowing stretched exponential relaxation of the protein–water thermal bath significantly improves the theory–experiment agreement when applied to solid protein samples studied by Mössbauer spectroscopy.
中文翻译:

遍历性破坏血红素蛋白中铁的置换
我们提出了蛋白质中原子位移的动态转变模型。较高温度下均方位移的增加是由于蛋白质-水热浴中的静电力和范德华力使原子/分子位移的力常数变弱所致。当力-力相关函数的弛豫时间随着温度的升高而进入仪器观测窗口时,位移软化将经历非遍历的动态过渡。确定了两个交叉温度。目前与玻璃化转变有关的较低的交叉与水分子的旋转动态解冻有关,而水分子的旋转在被蛋白质带电表面残基极化的纳米域内。较高的交叉温度,通常分配给动态过渡,标志着水平移的开始。所有的交叉都是遍历遍历的过渡,这取决于相应的观察窗口。当将蛋白质-水热浴拉伸到指数范围时,当将其应用于由Mössbauer光谱学研究的固体蛋白质样品时,可以大大改善理论-实验的一致性。
更新日期:2017-11-15
中文翻译:

遍历性破坏血红素蛋白中铁的置换
我们提出了蛋白质中原子位移的动态转变模型。较高温度下均方位移的增加是由于蛋白质-水热浴中的静电力和范德华力使原子/分子位移的力常数变弱所致。当力-力相关函数的弛豫时间随着温度的升高而进入仪器观测窗口时,位移软化将经历非遍历的动态过渡。确定了两个交叉温度。目前与玻璃化转变有关的较低的交叉与水分子的旋转动态解冻有关,而水分子的旋转在被蛋白质带电表面残基极化的纳米域内。较高的交叉温度,通常分配给动态过渡,标志着水平移的开始。所有的交叉都是遍历遍历的过渡,这取决于相应的观察窗口。当将蛋白质-水热浴拉伸到指数范围时,当将其应用于由Mössbauer光谱学研究的固体蛋白质样品时,可以大大改善理论-实验的一致性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号